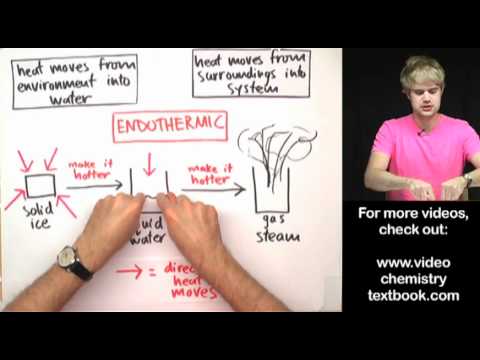
Q. Is water evaporating an exothermic process?
Evaporation is endothermic because water molecules must absorb heat from the surroundings to increase their kinetic energy.
Q. Is evaporation and endothermic process explain?
Evaporation is an endothermic process. The reaction in which heat is absorbed by the system is known as endothermic reaction. In evaporation heat is absorbed and passed to the water which forms vapor when heated. Reason : It absorbs heat from the surface and causes cooling.
Table of Contents
- Q. Is water evaporating an exothermic process?
- Q. Is evaporation and endothermic process explain?
- Q. Is evaporation an endothermic or exothermic process give an example to support your answer?
- Q. What type of process is evaporation?
- Q. What type of process is evaporation exothermic?
- Q. Is sublimation endothermic or exothermic?
- Q. What’s the opposite of evaporation?
- Q. Can pure water exist as a liquid at 110 C?
- Q. What happens to water under extreme pressure?
- Q. Why is 32 F freezing?
- Q. How fast will water freeze at 0 degrees?
- Q. How cold is water under ice?
- Q. Do fish die in a frozen lake?
- Q. Do fish die when water freezes?
- Q. What happens to water under ice?
Q. Is evaporation an endothermic or exothermic process give an example to support your answer?
✔Evaporation is a endothermic reaction because we know that evaporation cause cooling and carry heat from surrounding to increase kinetic energy of water molecules .
Q. What type of process is evaporation?
Evaporation is one of the two forms of vaporization. It is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to enter the gaseous state.
Q. What type of process is evaporation exothermic?
Evaporation is endothermic. For condensation the molecules are giving up their heat energy. When molecules give up heat energy, it is called exothermic.
Q. Is sublimation endothermic or exothermic?
Hence, freezing, condensation, and deposition are all exothermic phase transitions….Phases and Phase Transitions.
| Phase Transition | Direction of ΔH |
|---|---|
| Sublimation (solid to gas) | ΔH>0; enthalpy increases (endothermic process) |
Q. What’s the opposite of evaporation?
The opposite of evaporation is condensation. While evaporation is the process of liquid changing into a gas, condensation is the process of a gas…
Q. Can pure water exist as a liquid at 110 C?
Yes, pure liquid water can exist at 110°C. More specifically, water boils at 100°C at a pressure of 1 atm. If the pressure was lower, the temperature needed to boil water would be higher.
Q. What happens to water under extreme pressure?
“Compressing water customarily heats it. But under extreme compression, it is easier for dense water to enter its solid phase [ice] than maintain the more energetic liquid phase [water].” Ice is odd. Most things shrink when they get cold, and so they take up less space as solids than as liquids.
Q. Why is 32 F freezing?
The freezing temperature of water is 32 degrees Fahrenheit because of the unique characteristics of the water molecule, H2O. For water, this happens at 212 degrees Fahrenheit. Freezing happens when the molecules of a liquid get so cold that they slow down enough to hook onto each other, forming a solid crystal.
Q. How fast will water freeze at 0 degrees?
So, how long does it take for water to freeze? In a freezer, it will take from 1 hour to two hours if you what to get ice cubes at a temperature of 0° F. If your water is cold or really hot, the water will freeze even faster (around 45 minutes).
Q. How cold is water under ice?
Most of the water under the ice is 39 Fahrenheit; however, there is a thin layer of water under the ice that is colder than 39 and therefore less dense.
Q. Do fish die in a frozen lake?
When an entire lake becomes oxygen starved, winter-kill events take place. As the anoxic zone creeps upwards into the water column, fish cling to the under-surface of the ice as the oxygen is depleted, until they suffocate to death.
Q. Do fish die when water freezes?
In shallow lakes that freeze almost to the bottom, fish kills can happen when there is not enough oxygen left in the water. However, colder water can hold more dissolved gas than warmer water can, so water below freezing holds the most oxygen. Then, because fish metabolism has slowed down, they are using less oxygen.
Q. What happens to water under ice?
Right when the water freezes to ice, the ice becomes significantly less dense than the water and continues to float on the lake’s surface. Below 4° Celsius, water becomes less dense as it gets colder, causing water about to freeze to float to the top. Public Domain Image, source: Christopher S. Baird.