Electrostatic interaction (van der Waals interaction): The attractive or repulsive interaction between objects having electric charges. Electrostatic attraction (shown in red) between the δ+ and δ- ends of a polar covalent N-H bond allow for hydrogen bonding and base pairing within the DNA double helix.
Q. What is electrostatic force Class 8?
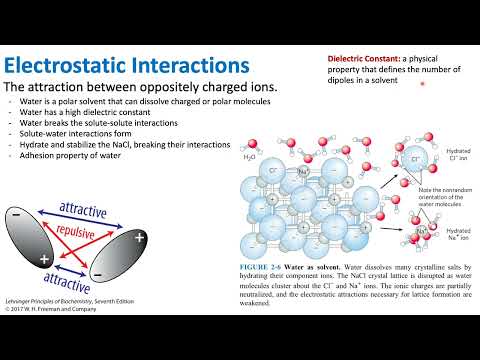
Electrostatic force is the force exerted by a charged body on another charged or uncharged body. This is because the comb is charged because of the rubbing on your hair and it attracts uncharged objects like paper.
Table of Contents
- Q. What is electrostatic force Class 8?
- Q. What is another word for electrostatic?
- Q. Are electrostatic interactions weak?
- Q. What is required for an electrostatic interaction?
- Q. How do you break electrostatic interactions?
- Q. Are charge charge interactions electrostatic interactions?
- Q. What is the strongest noncovalent interaction between hydrocarbons?
- Q. Which Noncovalent interaction is considered to be the weakest?
Q. What is another word for electrostatic?
In this page you can discover 15 synonyms, antonyms, idiomatic expressions, and related words for electrostatic, like: electrodynamic, static, biostatic, geostatic, precipitator, coulomb, exciton, ionization, dielectric, electrostatics and ferromagnetic.
Q. Are electrostatic interactions weak?
Hydrogen bonds are fundamentally electrostatic interactions. Hydrogen bonds are much weaker than covalent bonds. They have energies of 1–3 kcal mol-1 (4–13 kJ mol-1) compared with approximately 100 kcal mol-1 (418 kJ mol-1) for a carbon-hydrogen covalent bond.
Q. What is required for an electrostatic interaction?
Definition. The electrostatic interactions appear universally if charges (either positive or negative) become separated by a finite distance due to ionization or attachment of ionic species. Thus, there appear repulsive forces between like charges and attractive for opposite charges.
Q. How do you break electrostatic interactions?
If you speak of a complex, then you can try to increase the ionic strength of the solution in a controlled manner, in order to weaken the electrostatic interactions, or change the pH. You can also use urea or guanidine, always under controlled conditions, for the non-electrostatic interactions.
Q. Are charge charge interactions electrostatic interactions?
All intramolecular and intermolecular interactions are the result of electrostatic interactions between charged particles. The potential energy surface of the interaction of two charged particles, calculated using Coulomb’s law. …
Q. What is the strongest noncovalent interaction between hydrocarbons?
London dispersion forces
Q. Which Noncovalent interaction is considered to be the weakest?
London dispersion forces are the weakest type of non-covalent interaction.