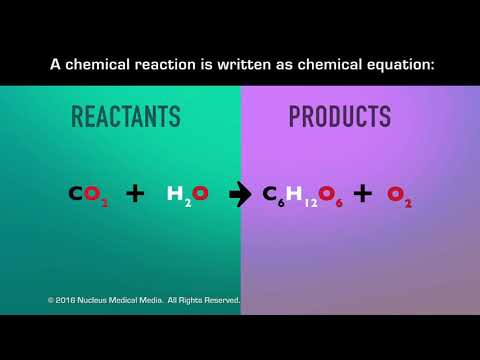
Q. What are new substances formed in a chemical reaction?
A chemical reaction is the process in which atoms present in the starting substances rearrange to give new chemical combinations present in the substances formed by the reaction. These starting substances of a chemical reaction are called the reactants, and the new substances that result are called the products.
Q. What are molecules in a chemical equation?
molecule: The smallest particle of a specific compound that retains the chemical properties of that compound; two or more atoms held together by chemical bonds.
Table of Contents
- Q. What are new substances formed in a chemical reaction?
- Q. What are molecules in a chemical equation?
- Q. Why are new substances formed in a chemical reaction?
- Q. What does a chemical reaction always produce?
- Q. What are the 6 types of chemical reactions?
- Q. What are the major types of chemical reactions?
- Q. What are the different types of chemical reactions with examples?
- Q. What is a simple decomposition reaction?
- Q. What are different types of decomposition reaction?
- Q. What is decomposition reaction and its types?
- Q. What type of chemical reaction is water?
- Q. What is the chemical formula of oxygen?
- Q. What are the 4 types of chemical reactions?
- Q. What is the chemical equation for oxygen?
- Q. What is the chemical formula for methane?
- Q. What is the chemical reaction of hydrogen gas?
- Q. What is the chemical name of rust?
- Q. Is rust a Colour?
- Q. Is the chemical name of rust is iron III oxide?
- Q. What is Fe2O3 made of?
- Q. What prevents rusting?
- Q. Why is iron oxide brittle?
Q. Why are new substances formed in a chemical reaction?
A chemical reaction happens when substances break apart or combine to form one or more new substances. New substances form when bonds break and new bonds form. The chemical properties of the new substances are different from those of the original substances. Some chemical reactions produce a precipitate.
Q. What does a chemical reaction always produce?
Chemical reactions involve breaking chemical bonds between reactant molecules (particles) and forming new bonds between atoms in product particles (molecules). The number of atoms before and after the chemical change is the same but the number of molecules will change.
Q. What are the 6 types of chemical reactions?
Six common types of chemical reactions are: synthesis, decomposition, single-displacement, double-displacement, combustion and acid-base reactions. Scientists classify them based on what happens when going from reactants to products.
Q. What are the major types of chemical reactions?
The five basic types of chemical reactions are combination, decomposition, single-replacement, double-replacement, and combustion.
Q. What are the different types of chemical reactions with examples?
The 5 primary types of chemical reactions are: Combination reaction. Decomposition reaction….
- Combination Reaction. A reaction in which two or more reactants combine to form a single product is known as a combination reaction.
- Decomposition Reaction.
- Displacement Reaction.
- Double Displacement Reaction.
- Precipitation Reaction.
Q. What is a simple decomposition reaction?
Summary. A decomposition reaction occurs when one reactant breaks down into two or more products. This can be represented by the general equation: AB → A + B. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
Q. What are different types of decomposition reaction?
Decomposition reactions can be classified into three types:
- Thermal decomposition reaction.
- Electrolytic decomposition reaction.
- Photo decomposition reaction.
Q. What is decomposition reaction and its types?
A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more elements or new compounds. These reactions often involve an energy source such as heat, light, or electricity that breaks apart the bonds of compounds. For example, CaCO3→CaO+CO2.
Q. What type of chemical reaction is water?
When two hydrogens and an oxygen share electrons via covalent bonds, a water molecule is formed. An example of a simple chemical reaction is the breaking down of hydrogen peroxide molecules, each of which consists of two hydrogen atoms bonded to two oxygen atoms (H2O2).
Q. What is the chemical formula of oxygen?
O2
Q. What are the 4 types of chemical reactions?
Representation of four basic chemical reactions types: synthesis, decomposition, single replacement and double replacement.
Q. What is the chemical equation for oxygen?
As we inspect this equation we see that there is one carbon atom on each side of the arrow and that there are two oxygen atoms on each side….5.2: Chemical Equations.
| Element | Chemical Formula |
|---|---|
| Hydrogen | H2 |
| Oxygen | O2 |
| Nitrogen | N2 |
| Fluorine | F2 |
Q. What is the chemical formula for methane?
CH₄
Q. What is the chemical reaction of hydrogen gas?
For example, hydrogen gas and ferrous oxide react, yielding metallic iron and water, H2 + FeO → Fe + H2O; hydrogen gas reduces palladium chloride to form palladium metal and hydrogen chloride, H2 + PdCl2 → Pd + 2HCl.
Q. What is the chemical name of rust?
iron oxide
Q. Is rust a Colour?
Rust is an orange-brown color resembling iron oxide. It is a commonly used color in stage lighting and appears roughly the same color as photographic safelights when used over a standard tungsten light source.
Q. Is the chemical name of rust is iron III oxide?
Fe2O3 is ferromagnetic, dark red, and readily attacked by acids. Iron(III) oxide is often called rust, and to some extent this label is useful, because rust shares several properties and has a similar composition. To a chemist, rust is considered an ill-defined material, described as hydrated ferric oxide.
Q. What is Fe2O3 made of?
Fe2O3 is the chemical formula of Iron(III) oxide which has three oxygen atoms, two iron atoms.
Q. What prevents rusting?
9 Ways to Prevent Rust
- Use an Alloy. Many outdoor structures, like this bridge, are made from COR-TEN steel to reduce the effects of rust.
- Apply Oil.
- Apply a Dry Coating.
- Paint the Metal.
- Store Properly.
- Galvanize.
- Blueing.
- Powder Coating.
Q. Why is iron oxide brittle?
The main catalyst for the rusting process is water. Iron or steel structures might appear to be solid, but water molecules can penetrate the microscopic pits and cracks in any exposed metal. As the atoms combine, they weaken the metal, making the structure brittle and crumbly.