Q. What are the first 10 alkenes?
List of Alkenes
- Ethene (C2H4)e.
- Propene (C3H6)
- Butene (C4H8)
- Pentene (C5H10)
- Hexene (C6H12)
- Heptene (C7H14)
- Octene (C8H16)
- Nonene (C9H18)
Q. What are the first 10 straight chain alkanes?
Terms in this set (10)
Table of Contents
- Q. What are the first 10 alkenes?
- Q. What are the first 10 straight chain alkanes?
- Q. What is the name for a ten carbon continuous chain alkane?
- Q. What is the molecular formula of an alkane with 10 carbon atoms?
- Q. What are 20 carbon chains?
- Q. What are the first 10 alkyne?
- Q. What is a ten carbon chain called?
- Q. How do you remember the first 10 alkanes?
- Q. What are the first 10 hydrocarbons?
- Q. What is an 11 carbon alkane called?
- Q. What is the alkene formula?
- Q. What is the formula of alkane alkene alkyne?
- Q. How are root names used in alkane graph?
- Q. How are alkanes named according to the number of carbons?
- Q. What is the formula for straight chain alkanes?
- Q. How are double bonds indicated in alkenes hydrocarbons?
- methane. CH4 (C)
- ethane. C2H6 (C-C)
- propane. C3H8 (C-C-C)
- butane. C4H10 (C-C-C-C)
- pentane. C5H12 (C-C-C-C-C)
- hexane. C6H14 (C-C-C-C-C-C)
- heptane. C7H16 (C-C-C-C-C-C-C)
- octane. C8H18 (C-C-C-C-C-C-C-C)
Q. What is the name for a ten carbon continuous chain alkane?
Straight-Chain and Branched Alkanes
| Table 1. Summary of the Straight-Chain Alkanes | ||
|---|---|---|
| Name | Formula | Melting Point (°C) |
| Octane | C8H18 | -56.8 |
| Nonane | C9H20 | -51 |
| Decane | C10H22 | -29.7 |
Q. What is the molecular formula of an alkane with 10 carbon atoms?
Decane
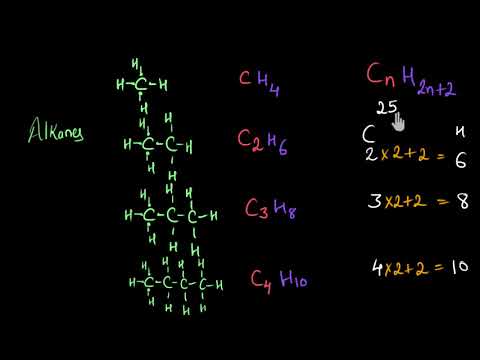
The general formula for the alkanes is C nH 2n+2 (where n is the number of carbon atoms in the molecule). Decane is an alkane. Its molecules contain 10 carbon atoms .
Q. What are 20 carbon chains?
List of straight-chain alkanes
| Number of C atoms | Number of isomers | Name of straight chain |
|---|---|---|
| 18 | 60523 | n-octadecane |
| 19 | 148284 | n-nonadecane |
| 20 | 366319 | n-icosane |
| 21 | 910726 | n-henicosane |
Q. What are the first 10 alkyne?
Here are the molecular formulas and names of the first ten carbon straight chain alkynes….Introduction.
| Name | Molecular Formula |
|---|---|
| Propyne | C3H4 |
| 1-Butyne | C4H6 |
| 1-Pentyne | C5H8 |
| 1-Hexyne | C6H10 |
Q. What is a ten carbon chain called?
A two-carbon chain is called ethane; a three-carbon chain, propane; and a four-carbon chain, butane. Longer chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10).
Q. How do you remember the first 10 alkanes?
For example the first 10 alkanes in order are , Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane and Decane. These can be memorised with “Many elephants prefer blue pinapples.
Q. What are the first 10 hydrocarbons?
Terms in this set (10)
- methane. CH₄
- ethane. C₂H₆
- propane. C₃H₈
- butane. C₄H₁₀
- pentane. C₅H₁₂
- hexane. C₆H₁₄
- heptane. C₇H₁₆
- octane. C₈H₁₈
Q. What is an 11 carbon alkane called?
undecane (11 carbons) dodecane (12 carbons) tridecane (13 carbons) tetradecane (14 carbons)
Q. What is the alkene formula?
The general formula for the alkenes is C nH 2n, where n is the number of carbon atoms in the molecule. Worked example. Decene is an alkene. Its molecules contain 10 carbon atoms .
Q. What is the formula of alkane alkene alkyne?
Alkanes have the general formula of CnH2n+2 where n is the number of carbon atoms. Alkenes have the general formula CnH2n. The general formula for alkynes is CnH2n-2. Acetylene is the simplest alkyne with the formula as C2H2.
Q. How are root names used in alkane graph?
Root names give the number of carbons in the longest continuous chain. Root names are used with various “endings” to indicate branches, type of bonds between carbons, and functional groups. The following list gives the most basic root the with normal hydrocarbon alkane endings for the number of carbons in the longest continuous chain.
Q. How are alkanes named according to the number of carbons?
Alkanes that have 5 or more carbons are named using prefixes that indicate the number of carbons. So, pent- means 5, hex- means 6, hept- means 7, and so on. The simple branched alkanes have prefixes on their names to distinguish them from the linear alkanes.
Q. What is the formula for straight chain alkanes?
Straight-chain alkanes have the formula CnH2n+2 Carbons are sp3 hybridized
Q. How are double bonds indicated in alkenes hydrocarbons?
Alkenes and Alkynes – unsaturated hydrocarbons. Double bonds in hydrocarbons are indicated by replacing the suffix -ane with -ene. If there is more than one double bond, the suffix is expanded to include a prefix that indicates the number of double bonds present (-adiene, -atriene, etc.).