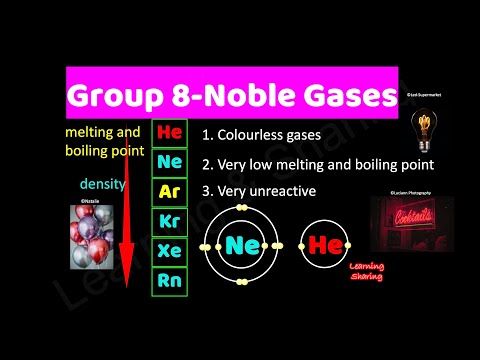
Group 8A — The Noble or Inert Gases. Group 8A (or VIIIA) of the periodic table are the noble gases or inert gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The name comes from the fact that these elements are virtually unreactive towards other elements or compounds.
Q. What is the 3 types of elements?
Elements in different groups are lumped together in one of three classes, depending on their properties. The classes are metals, nonmetals, and metalloids. Knowing the class of an element lets you predict many of its properties.
Table of Contents
- Q. What is the 3 types of elements?
- Q. What is the difference between group A and group B metals?
- Q. What are the first three elements in Group 8?
- Q. Why are there 3 columns in subgroup Viiib?
- Q. How many valence electrons does group 12 have?
- Q. Why Valency of oxygen is 2 in water?
- Q. Why is the Valency of oxygen negative?
- Q. How many shells are in oxygen?
- Q. What is the variable valency of oxygen?
- Q. Why the Valency of Sulphur is 4?
- Q. Does Oxygen show variable Covalency?
- Q. What is the variable valency of gold?
Q. What is the difference between group A and group B metals?
What is the basic difference between group A and group B elements from the electronic configuration point of view. In group A elements, the added electrons goes to the outermost shell. In group B, the additional electron goes to penultimate shell.
Q. What are the first three elements in Group 8?
Group 8 is a group (column) of chemical elements in the periodic table. It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals.
Q. Why are there 3 columns in subgroup Viiib?
True of alkali metals. There are three columns in Subgroup VIIIB because iron, cobalt, and nickel have similar properties.
Q. How many valence electrons does group 12 have?
3 valence electrons
Q. Why Valency of oxygen is 2 in water?
Valency is the number of particular atoms combined with or displaced with another atom to form a compound. Water which is H 2O, two atoms of hydrogen combine with one atom of oxygen. The valency of oxygen is 2, because it needs two atoms of hydrogen to form water.
Q. Why is the Valency of oxygen negative?
The valency of oxygen is -2. This means oxygen needs to gain or share two electrons for stability. Remember, electrons are negative, which is why the…
Q. How many shells are in oxygen?
So… for the element of OXYGEN, you already know that the atomic number tells you the number of electrons. That means there are 8 electrons in an oxygen atom. Looking at the picture, you can see there are two electrons in shell one and six in shell two. ► More about the history and places to find oxygen.
Q. What is the variable valency of oxygen?
2
Q. Why the Valency of Sulphur is 4?
It has 6 atoms in the outermost shell and it needs only two electrons from another atom in bonding to complete its octet. For two atoms of oxygen we need in total four electrons. Therefore, in this case in SO2 molecule the valency of sulphur is +4.
Q. Does Oxygen show variable Covalency?
The term Covalency is only for smaller elements which can form covalent because the larger elements show variable valency , so for them valency term is replaced by oxidation state or number. i.e of period 1, 2 of p- block . For Oxygen Covalency is 3 in O3 . For Fluorine Covalency is 1 .
Q. What is the variable valency of gold?
Gold (Au) has two main valences 1 and 3. But due to his noble character, it does not react with other atoms.