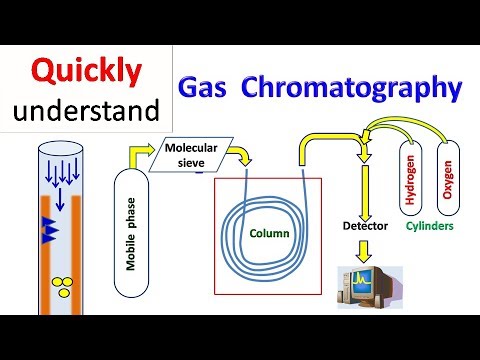
Q. What are the major advantages of gas chromatography over TLC?
Wide choice of samples Gas chromatography is ideally suited for analysis of volatile compounds. Samples with boiling points as high as 380 to 400°C can be analysed by temperature programming. The samples can range from liquids, gases and dissolved solids. TLC techniques, however, cannot be used for analysis of gases.
Q. Why is TLC more accurate than paper chromatography?
TLC tends to produce more useful chromatograms than paper chromatography, which show greater separation of the components in the mixture – and are therefore easier to analyse. The distance a sample travels can depend on the size or the polarity of the molecules involved.
Table of Contents
- Q. What are the major advantages of gas chromatography over TLC?
- Q. Why is TLC more accurate than paper chromatography?
- Q. Why gas liquid chromatography is better than gas solid chromatography?
- Q. What is principle of gas solid chromatography?
- Q. Why gas solid chromatography is not commonly used?
- Q. Why is gas solid chromatography used?
- Q. What is the mobile phase in gas solid chromatography?
- Q. Which GC detector is destructive type?
- Q. Why can’t humans see UV light?
- Q. What the human eye Cannot see?
- Q. What light is invisible to the human eye?
- Q. What are the 3 uses of visible light?
- Q. Which color has the highest energy?
- Q. How do humans use light?
Q. Why gas liquid chromatography is better than gas solid chromatography?
In Gas liquid chromatography, the retention time (Rf) is relatively short when compared to that of GSC. Relatively very small concentrations of the samples can be used in Gas solid chromatography. Relatively higher concentration of samples can be used in gas liquid chromatography.
Q. What is principle of gas solid chromatography?
GSC is a type of GC in which the same material acts as both the stationary phase and the support. 7. In this method, chemicals are retained by their adsorption to the surface of the support. This support is often an inorganic material such as silica or alumina.
Q. Why gas solid chromatography is not commonly used?
Gas solid chromatography is not used widely because of limited number of stationary phases available. In Gas solid chromatography, the principle of separation is adsorption. It’s mainly used for solutes which having less solubility in stationary phase.
Q. Why is gas solid chromatography used?
Gas-solid chromatography is now an important analytical tool for the separation and identification of various mixtures, from hydrogen isotopes and isomers to high boiling point substances.
Q. What is the mobile phase in gas solid chromatography?
Principle. Gas chromatography (GC) is the common name for chromatographic methods in which the mobile phase is gas, and the stationary phase is solid or liquid (gas–solid chromatography (GSC) or gas–liquid chromatography (GLC)). There are limitations to the type of compound suitable for GC analysis.
Q. Which GC detector is destructive type?
Destructive detectors In gas chromatography: Flame ionization detector (FID) Flame photometric detector (FPD) Nitrogen Phosphorus Detector (NPD)
Q. Why can’t humans see UV light?
aThe human eye can see light with wavelengths between 380 and 700 nanometers. cMost humans cannot see ultraviolet light because it has a shorter wavelength than violet light, putting it outside of the visible spectrum.
Q. What the human eye Cannot see?
The human eye can only see visible light, but light comes in many other “colors”—radio, infrared, ultraviolet, X-ray, and gamma-ray—that are invisible to the naked eye. On one end of the spectrum there is infrared light, which, while too red for humans to see, is all around us and even emitted from our bodies.
Q. What light is invisible to the human eye?
Visible light
Q. What are the 3 uses of visible light?
Aside from sight, there are other important uses for visible light. We concentrate visible light to make lasers to use in everything from surgery, to CD players to laser pointers. Visible light waves also make our TV, computer and cell phone screens work.
Q. Which color has the highest energy?
violet
Q. How do humans use light?
We use it to communicate, navigate, learn and explore. Light is far more than just what we can detect with our eyes. It takes the form of radio waves, microwaves, infrared, ultraviolet, X-rays and gamma rays.