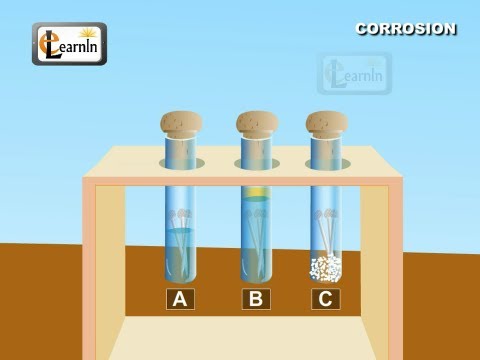
So, the presence of air and water vapor in air are two necessary conditions for the rusting of iron.
Q. What is Fe2O3 xH2O?
Fe2O3. xH2O is usually called hydrated ferric Oxide. It is a hydrated crystal. When Ferrous ion forms an ionic bond with Oxygen, it is called Ferrous Oxide.
Table of Contents
- Q. What is Fe2O3 xH2O?
- Q. What are the conditions required for rusting?
- Q. What are the conditions necessary for rusting How can we prevent rusting?
- Q. What three things are required to corrode metal?
- Q. Which metal will corrode fast?
- Q. What are the 3 types of corrosion?
- Q. How quickly can corrosion occur?
- Q. What are the methods to prevent corrosion?
- Q. Is there anything that seems to make rust form slower?
- Q. Does heat speed up rusting?
- Q. How long does it take for iron to rust underwater?
- Q. Does Zinc rust in water?
- Q. Does Zinc rust in salt water?
- Q. Why does zinc not rust?
- Q. Which is stronger aluminum or zinc?
- Q. What does zinc do to aluminum?
- Q. What is the easiest metal to cast?
- Q. Is magnesium stronger than titanium?
- Q. What is the strongest metal in the world?
- Q. What is the strongest alloy ever made?
- Q. What is the strongest alloy metal on earth?
- Q. What is the strongest thing on earth?
- Q. What metal is bulletproof?
- Q. What is the hardest metal to melt?
Q. What are the conditions required for rusting?
Rusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron(III) oxide, which we see as rust. Iron and steel rust when they come into contact with water and oxygen – both are needed for rusting to occur.
Q. What are the conditions necessary for rusting How can we prevent rusting?
Rusting of iron takes place in the presence of moisture and air. So the presence of air and water vapour in air are two necessary conditions for rusting of iron.
Q. What three things are required to corrode metal?
Corrosion is a two-step process that requires three things: a metallic surface, an electrolyte, and oxygen. During the corrosion process, surface-level metal atoms dissolve into an aqueous solution, leaving the metal with an excess of negative charge. The resultant ions are removed by a suitable electron acceptor.
Q. Which metal will corrode fast?
We know that plain carbon steel corrodes faster in water than stainless steel.
Q. What are the 3 types of corrosion?
Eight Forms of Corrosion
- Uniform Attack. Uniform attack is the most common form of corrosion.
- Galvanic or Two-Metal Corrosion.
- Crevice Corrosion.
- Pitting.
- Intergranular Corrosion.
- Selective leaching.
- Erosion Corrosion.
- Stress-corrosion cracking.
Q. How quickly can corrosion occur?
Steel corrodes quickly in acidic environments and slowly or not at all as alkalinity is increased. The corrosion rate of steel in soil can range from less than 0.2 microns per year in favorable conditions to 20 microns per year or more in very aggressive soils.
Q. What are the methods to prevent corrosion?
How to prevent corrosion
- Choose the right Metal Type. One of the simplest ways to prevent corrosion is to use a corrosion resistant metal such as stainless steel, duplex, super duplex, nickel alloy or 6% Moly.
- Protective Coatings.
- Environmental Measures.
- Sacrificial Coatings.
- Corrosion Inhibitors.
- Metal Plating.
- Design Modification.
Q. Is there anything that seems to make rust form slower?
Stainless steel has added nickel and chromium which bind to the iron atoms and keep them from oxidizing. I haven’t seen stainless steel rust even over long periods of time. 2) The iron can be painted or coated with oil, preventing oxygen and water from coming into contact. This can slow or halt rusting.
Q. Does heat speed up rusting?
Heat increases the rate of chemical reactions, which means hot iron rusts quicker than cold iron. Auto parts in places of extreme heat rust quickly unless protected by a non-rusting material.
Q. How long does it take for iron to rust underwater?
it will start almost immediately, and may progress at a rate of around 1/2mm per year. This rate will vary depending on the alloy and water conditions.
Q. Does Zinc rust in water?
Like all ferrous metals, zinc corrodes when exposed to air and water. The zinc is protected by the formation of a patina layer on the surface of the coating. The patina layer is the products of zinc corrosion and rust.
Q. Does Zinc rust in salt water?
The aluminum, bronze and iron parts in the saltwater undergo less corrosion. Zinc anodes are the preferred choice in metal alloys for saltwater applications that need a sacrificial anode, because the alloy is less resistant to the saltwater’s electrolytes.
Q. Why does zinc not rust?
The zinc layer acts as a sacrificial metal for the steel. This means that the zinc layer will combine with the oxygen more readily than the iron in the steel will. This creates a zinc oxide layer that prevents the formation of iron oxide, thus eliminating the possibility of rust forming.
Q. Which is stronger aluminum or zinc?
Longer Tool Life: Simply put, zinc is much stronger and more durable than aluminum. Lower Cost: Zinc materials are initially more costly than aluminum because this alloy is of a higher quality. But zinc die casting prices are generally lower than those of aluminum.
Q. What does zinc do to aluminum?
Zinc (Zn) 7xxx – The addition of zinc to aluminum (in conjunction with some other elements, primarily magnesium and/or copper) produces heat-treatable aluminum alloys of the highest strength. The zinc substantially increases strength and permits precipitation hardening.
Q. What is the easiest metal to cast?
Copper alloys
Q. Is magnesium stronger than titanium?
Titanium is significantly stronger than both aluminium and magnesium, although its higher density means that strength-to-weight ratios for the three metals tend to be similar. It is often the first port of call for engineers looking to replace steel in a lightweighting exercise for stressed components.
Q. What is the strongest metal in the world?
tungsten
Q. What is the strongest alloy ever made?
- The Strongest Natural Metal: Tungsten. As far as pure metals go, tungsten has the highest tensile strength, with an ultimate strength of 1510 megapascals.
- The Strongest Alloy: Steel.
- The Hardest Metal: Chromium.
- The Most Useful Strong Metal: Titanium.
Q. What is the strongest alloy metal on earth?
Steel
Q. What is the strongest thing on earth?
The World’s Strongest Stuff
- Diamond. Unmatched in its ability to resist being scratched, this much-loved gemstone ranks the highest in terms of hardness.
- Graphene.
- Spider silk.
- Carbon/carbon composite.
- Silicon carbide.
- Nickel-based super-alloys.
Q. What metal is bulletproof?
Created by melting aluminum around hollow metal spheres, composite metal foam is 70% lighter than sheet metal and can absorb 80 times more energy than steel. It is fireproof, radiation-resistant, and even bulletproof.