There are 3 types of point defects: Stoichiometric defect. Frenkel defect. Schottky defect.
Q. What is slip deformation?
Slip is the prominent mechanism of plastic deformation in metals. It involves sliding of blocks of crystal over one other along definite crystallographic planes, called slip planes. • it is analogous to a deck of cards when it is pushed from one end. Slip occurs when shear stress applied exceeds a critical value.
Table of Contents
- Q. What is slip deformation?
- Q. What is slip in material science?
- Q. How do defects affect material properties?
- Q. Is the presence of defects in a material good or bad?
- Q. What are the types of crystal defects?
- Q. What does surface mean?
- Q. How do you explain surface?
- Q. What’s the meaning of embedded?
- Q. What does surface tension mean?
- Q. What does surface tension depend on?
- Q. What factors affect surface tension?
- Q. What is surface tension caused by?
- Q. Does salt water increase surface tension?
- Q. Which has more surface tension water or oil?
- Q. Does surface tension increase with temperature?
- Q. What is the effect on surface tension of temperature?
- Q. How does surface tension vary with temperature?
- Q. What is effect of impurity on surface tension?
Q. What is slip in material science?
In materials science, slip is the large displacement of one part of a crystal relative to another part along crystallographic planes and directions. An external force makes parts of the crystal lattice glide along each other, changing the material’s geometry.
Q. How do defects affect material properties?
Line defects weakens the structure along a one-dimensional space, and the defects type and density affects the mechanical properties of the solids. Thus, formation and study of dislocations are particularly important for structural materials such as metals. This link gives some impressive images of dislocations.
Q. Is the presence of defects in a material good or bad?
Certainly, defects can limit what materials can do—for example, the theoretical strength calculated for crystalline solids is almost never observed in the laboratory because real materials generally contain a certain number of dislocations that can provide sites for the material to crack and fail.
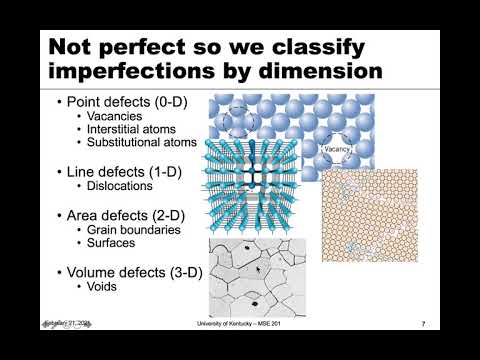
Q. What are the types of crystal defects?
- Point defects (vacancies, interstitial defects, substitution defects)
- Line defect (screw dislocation, edge dislocation)
- surface defects (material surface, grain boundaries)
- Substitutional – one atom is replaced by as different type of atom.
- Interstitial – extra atom is inserted into the lattice structure at a.
Q. What does surface mean?
(Entry 1 of 3) 1 : the exterior or upper boundary of an object or body on the surface of the water the earth’s surface. 2 : a plane or curved two-dimensional locus of points (such as the boundary of a three-dimensional region) plane surface surface of a sphere.
Q. How do you explain surface?
the outer face, outside, or exterior boundary of a thing; outermost or uppermost layer or area. any face of a body or thing: the six surfaces of a cube. extent or area of outer face; superficial area. the outward appearance, especially as distinguished from the inner nature: to look below the surface of a matter.
Q. What’s the meaning of embedded?
transitive verb. 1a : to enclose closely in or as if in a matrix fossils embedded in stone. b : to make something an integral part of the prejudices embedded in our language. c : to prepare (a microscopy specimen) for sectioning by infiltrating with and enclosing in a supporting substance.
Q. What does surface tension mean?
Surface Tension: “The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules.” The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.
Q. What does surface tension depend on?
Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension.
Q. What factors affect surface tension?
Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends on the nature of the liquid, the surrounding environment and temperature. Liquids where molecules have large attractive intermolecular force will have a large surface tension.
Q. What is surface tension caused by?
The surface tension arises due to cohesive interactions between the molecules in the liquid. At the bulk of the liquid, the molecules have neighboring molecules on each side. Molecules are pulling each other equally at all directions causing a net force of zero.
Q. Does salt water increase surface tension?
Compounds that lower water’s surface tension are called surfactants, which work by separating the water molecules from one another. Adding salt to water does increase the surface tension of water, although not by any significant amount. …
Q. Which has more surface tension water or oil?
Water has a high surface tension (72 dynes/cm). Oil differs from water in many respects, the most important of which is surface tension. Oil has a surface tension of 30–35 dynes/cm, meaning that oil-soluble fatty surfactants do not provide the desired surface tension reduction for oils.
Q. Does surface tension increase with temperature?
As temperature decreases, surface tension increases. Conversely, as surface tension decreases strong; as molecules become more active with an increase in temperature becoming zero at its boiling point and vanishing at critical temperature.
Q. What is the effect on surface tension of temperature?
As the temperature increases, viscosity decreases. Surface tension decreases with increase in the temperature. So these, are the effects of temperature on viscosity and surface tension.
Q. How does surface tension vary with temperature?
Increase in temperature increases the kinetic energy of the molecules and effectiveness of intermolecular attraction decreases, so surface tension decreases as the temperature is raised.
Q. What is effect of impurity on surface tension?
As the concentration of the impurity increases the equilibrium surface tension decreases. The decrease in the equilibrium surface tension is also dependent upon the concentration of the tetradecylpyridinium bromide. As the concentration of the surfactant (TPB) increases the effect of the impurity is reduced.