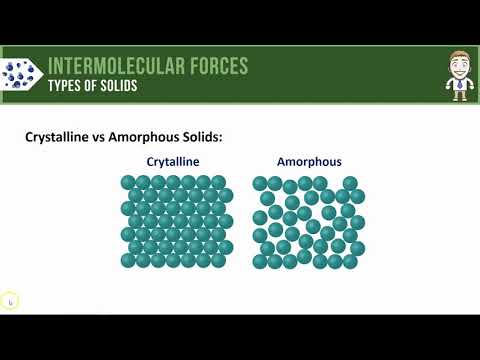
Solids can be classified into two types: crystalline and amorphous. Crystalline solids are the most common type of solid. They are characterized by a regular crystalline organization of atoms that confer a long-range order. Amorphous, or non-crystalline, solids lack this long-range order.
Q. Why do particles of solid Cannot move around?
Solid – In a solid, the attractive forces keep the particles together tightly enough so that the particles do not move past each other. The kinetic energy of the molecule is greater than the attractive force between them, thus they are much farther apart and move freely of each other.
Table of Contents
- Q. Why do particles of solid Cannot move around?
- Q. What are two characteristics of solid?
- Q. What is the features of solid?
- Q. What type of solid is i2?
- Q. Is iodine is covalent solid?
- Q. Is Iodine is an atomic solid?
- Q. Is Iodine a network solid?
- Q. Which of following is network Solid?
- Q. Which is network of solid?
- Q. Which of the following is not a network solid?
- Q. Which of the following is an amorphous solid?
- Q. Which of the following is a network solid * 1 point?
- Q. Which of the following is an example of a crystalline solid?
- Q. Is AlN covalent solid?
- Q. Which of the following is crystal solid?
Q. What are two characteristics of solid?
Solids are defined by the following characteristics:
- definite shape (rigid)
- definite volume.
- particles vibrate around fixed axes.
Q. What is the features of solid?
Solid are characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does expands to fill the entire volume available to it like a gas .
Q. What type of solid is i2?
Iodine is an example of a molecular solid. Each iodine molecule is made up of 2 iodine atoms, held together by a strong covalent bond. Each iodine molecule is held to another by weak Van Der Waal’s forces. Low melting and boiling point due to weak forces between molecules.
Q. Is iodine is covalent solid?
Iodine: A simple covalent solid held together by van der Waals forces.
Q. Is Iodine is an atomic solid?
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a lustrous, purple-black non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius.
Q. Is Iodine a network solid?
Covalent solids are formed by networks or chains of atoms or molecules held together by covalent bonds. A perfect single crystal of a covalent solid is therefore a single giant molecule….Covalent Network Solids.
| Substance | ΔHsub (kJ/mol) | Average Bond Energy (kJ/mol) |
|---|---|---|
| iodine (s) | 62.42 | 149 |
Q. Which of following is network Solid?
– Diamond is made up of covalently bonded carbon atoms. We know that each carbon atom forms covalent bonds with other three carbon atoms and hence the resultant structure is called a network solid as it is spread in three dimensions in space. So, the correct answer is option (C) Diamond.
Q. Which is network of solid?
A network solid or covalent network solid is a chemical compound or element in which the atoms are bonded by covalent bonds in a continuous network extending throughout the material. In a network solid there are no individual molecules, and the entire crystal or amorphous solid may be considered a macromolecule.
Q. Which of the following is not a network solid?
the size of silicon is larger than the size of carbon. Hence, elemental silicon does not form a network solid like structure.
Q. Which of the following is an amorphous solid?
Glass is an amorphous solid.
Q. Which of the following is a network solid * 1 point?
Diamond is a network solid It is the hardest substance on Earth which has a high level of melting point. It has a structure of tetrahedral.
Q. Which of the following is an example of a crystalline solid?
Examples of crystalline solids include salt (sodium chloride), diamond, and sodium nitrate.
Q. Is AlN covalent solid?
AlN is chemical formula of aluminium nitride. it is an example of covalent solid.
Q. Which of the following is crystal solid?
Graphite is a crystalline form of the element, carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon under standard conditions. Was this answer helpful?