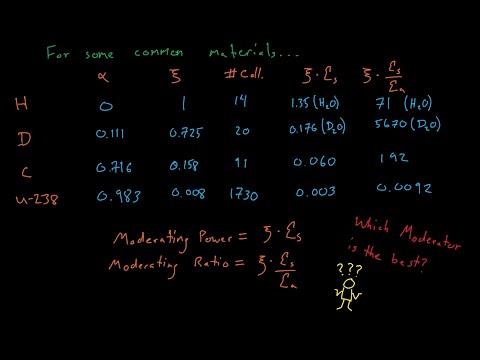Q. What causes a neutron to slow down?
If the collisions between neutrons and nuclei are elastic collisions, it implies that the closer in size the nucleus of an atom is to a neutron, the more the neutron will be slowed. For this reason, lighter elements tend to be more efficient moderators.
Q. Why are neutrons moderated to thermal speeds in nuclear reactors?
The moderator slows the fast (high-energy) neutrons emitted during fission to energies at which they are more likely to induce fission. In doing so, the moderator helps initiate and sustain a fission chain reaction.
Table of Contents
- Q. What causes a neutron to slow down?
- Q. Why are neutrons moderated to thermal speeds in nuclear reactors?
- Q. Which of the following is more suitable for slowing down the neutrons in a reactor?
- Q. What is the best neutron absorber?
- Q. What element absorbs neutrons?
- Q. What metal absorbs neutrons?
- Q. Why must neutrons be absorbed?
- Q. What happens to neutrons during beta decay?
- Q. What happens to a uranium 235 atom when it is bombarded with neutrons?
- Q. What is the difference between a stable and unstable atom?
Q. Which of the following is more suitable for slowing down the neutrons in a reactor?
Heavy water is very effective at slowing down (moderating) neutrons, giving CANDU reactors their important and defining characteristic of high “neutron economy”.
Q. What is the best neutron absorber?
Among the natural elements, boron, cadmium, and gadolinium are the best absorbers of slow neutrons by the capture process. It is believed that these heavier elements, and some isotopes of lighter elements, have been produced…
Q. What element absorbs neutrons?
Boron, in its bulk form, is an element that is used to control the rate of reactions in nuclear reactors by absorbing neutrons. Neutrons are uncharged particles that, along with protons (positively charged particles), make up the nucleus of atoms.
Q. What metal absorbs neutrons?
Control rods are used in nuclear reactors to control the fission rate of uranium or plutonium. Their compositions includes chemical elements, such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves fissioning.
Q. Why must neutrons be absorbed?
Neutron absorption by the nuclei of heavy elements gives rise to fission, in which heavy fragments, fast neutrons, and other radiations are released. Useful energy amounts to 190 MeV per fission, requiring only 1.3 g of U-235 to be consumed to obtain 1 MW ∙ d of thermal energy.
Q. What happens to neutrons during beta decay?
Beta decay occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other. In beta minus decay, a neutron decays into a proton, an electron, and an antineutrino: n Æ p + e – +.
Q. What happens to a uranium 235 atom when it is bombarded with neutrons?
When a free neutron hits the nucleus of a fissile atom like uranium-235 (235U), the uranium splits into two smaller atoms called fission fragments, plus more neutrons. Fission can be self-sustaining because it produces more neutrons with the speed required to cause new fissions. This creates the chain reaction.
Q. What is the difference between a stable and unstable atom?
An atom is stable if the forces among the particles that makeup the nucleus are balanced. An atom is unstable (radioactive) if these forces are unbalanced; if the nucleus has an excess of internal energy. Instability of an atom’s nucleus may result from an excess of either neutrons or protons.