To separate effectively the analytes, the characteristic used was the polarity of the NaCl sodium chloride. The evade solvents that are alike in polarity for the stationary stage are powerful draw to the paper fragments. The sodium chloride holds the same polarity of the stationary stage and could divide them out.
Q. Why are two solvents used in chromatography?
Why are two solvents used in the process? Different pigments will be soluble in one solvent but not another. Better separation of pigment bands will result if a combination of solvents is used.
Table of Contents
- Q. Why are two solvents used in chromatography?
- Q. Why is water not used in chromatography?
- Q. What makes your NaCl solution move up the paper?
- Q. How do you calculate Rf values?
- Q. Why Rf value has no units?
- Q. What does an RF value of 0 mean?
- Q. What affects Rf value?
- Q. Which pigment moves the fastest?
- Q. Which pigment has the lowest RF value?
Q. Why is water not used in chromatography?
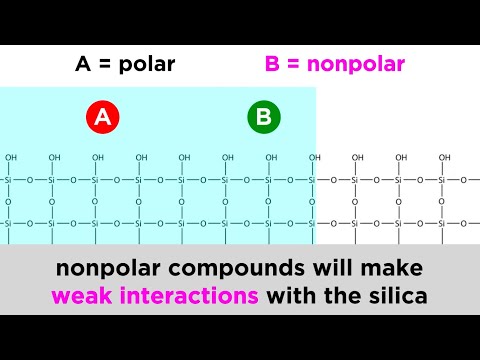
Answer. Explanation: It’s better to use a solvent that’s less polar, ethanol maybe, so that the non-polar compounds will travel up the paper, while the polar compounds stick to the paper, thus separating them out.
Q. What makes your NaCl solution move up the paper?
By using salt water instead of pure water, you will change how well the pigments are solubilized (how well they dissolve) in the liquid. If they are more soluble, they will travel farther because they would more rather stay in the liquid than interact (and sort of “stick”) to the paper.
Q. How do you calculate Rf values?
The Rf value of a compound is equal to the distance traveled by the compound divided by the distance traveled by the solvent front (both measured from the origin).
Q. Why Rf value has no units?
Rf values do not have units since it is a ration of distances. Because mixture solvents are often applied Rf values are usually written as the following examples: Rf = 0.66 (60% Ethanol) – if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water.
Q. What does an RF value of 0 mean?
did not move
Q. What affects Rf value?
In general, low polarity compounds have higher Rf values than higher polarity compounds. In general, the adsorptivity of compounds increases with increased polarity (i.e. the more polar the compound then the stronger it binds to the adsorbent). The eluting power of solvents increases with polarity.
Q. Which pigment moves the fastest?
pigment carotene
Q. Which pigment has the lowest RF value?
carotenes