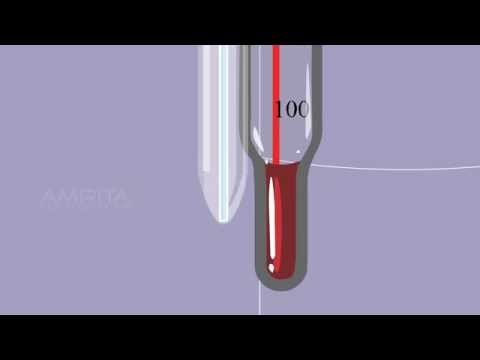
Q. What determines the boiling point of a liquid?
Types of Molecules: the types of molecules that make up a liquid determine its boiling point. If the intermolecular forces between molecules are: relatively strong, the boiling point will be relatively high. relatively weak, the boiling point will be relatively low.
Q. What properties affect boiling point?
The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. When the atmospheric pressure is equal to the vapor pressure of the liquid, boiling will begin.
Table of Contents
- Q. What determines the boiling point of a liquid?
- Q. What properties affect boiling point?
- Q. What are the observations at boiling point of water?
- Q. What molecular characteristics increase the boiling point of a pure liquid?
- Q. What increases boiling point?
- Q. What is Class 9 boiling point?
- Q. What is melting and boiling point?
- Q. What is boiling point of pure water?
- Q. What is Dry Ice Class 9?
- Q. Can you touch dry ice?
- Q. Is dry ice dangerous?
- Q. What is Dry Ice Class 8?
- Q. What are the properties of dry ice?
- Q. Why it is called dry ice?
- Q. What is dry ice samacheer Class 8 used for?
- Q. What is adolescence Class 8 samacheer?
- Q. What is green manure Class 8 samacheer?
- Q. What is heavier air or nitrogen?
- Q. Which air is heavier hot or cold?
- Q. Is nitrogen the same as oxygen?
- Q. Which gas is heavier than air?
- Q. Is a fart heavier than air?
- Q. What is the heaviest gas on Earth?
- Q. Does LPG rise or sink?
- Q. At what temperature does LPG become a gas?
- Q. Is methane lighter than air or heavier?
- Q. Can an LP gas leak ignite?
- Q. Can a propane leak kill you?
- Q. Can Propane cause carbon monoxide poisoning?
- Q. Is LP gas dangerous?
Q. What are the observations at boiling point of water?
Observations:
| Temperature when the water | Boiling point of water | |
|---|---|---|
| Starts boiling t1 (in °C) | Continue to boil (Stationary temperature) t2 (in °C) | (t1 + t2)/2 (in °C) |
Q. What molecular characteristics increase the boiling point of a pure liquid?
There are 3 important trends to consider.
- The relative strength of the four intermolecular forces is: Ionic > Hydrogen bonding > dipole dipole > Van der Waals dispersion forces.
- Boiling points increase as the number of carbons is increased.
- Branching decreases boiling point.
Q. What increases boiling point?
Compounds that can hydrogen bond will have higher boiling points than compounds that can only interact through London dispersion forces. An additional consideration for boiling points involves the vapor pressure and volatility of the compound. Typically, the more volatile a compound is, the lower its boiling point.
Q. What is Class 9 boiling point?
Boiling Point: The temperature at which the liquid boils and changes into gaseous state at the atmospheric pressure is called boiling point. For example, water boils at 100°C to form water vapour (at 76 cm pressure).
Q. What is melting and boiling point?
melting point is the temperature at which a solid changes into a liquid. boiling point is the temperature at which a liquid changes into a gas.
Q. What is boiling point of pure water?
212 °F
Q. What is Dry Ice Class 9?
Dry ice is the solid form of carbon dioxide. It is used primarily as a cooling agent. It is called dry ice because it sublimes and doesn’t leave any residue i.e. changes from solid to gaseous state without turning to liquid. Answer verified by Toppr.
Q. Can you touch dry ice?
Dry Ice temperature is extremely cold at -109.3°F or -78.5°C. Always handle Dry Ice with care and wear protective cloth or leather gloves whenever touching it. An oven mitt or towel will work. If touched briefly it is harmless, but prolonged contact with the skin will freeze cells and cause injury similar to a burn.
Q. Is dry ice dangerous?
Dry ice can be a very serious hazard in a small space that isn’t well-ventilated. As dry ice melts, it turns into carbon dioxide gas. In a small space, this gas can build up. If enough carbon dioxide gas is present, a person can become unconscious, and in some cases, die.
Q. What is Dry Ice Class 8?
Dry ice is solidified carbon dioxide. It is named so because it looks like ice and is way colder than ordinary ice. It stays dry because it directly converts to gaseous carbon dioxide without an intermediate liquid stage. Hence it is useful in storing edible things like ice cream which may get spoilt by water.
Q. What are the properties of dry ice?
Physical properties
| Property | Value |
|---|---|
| Appearance | Colorless, odorless gas; colorless liquid, or white opaque solid (dry ice) |
| Density Solid (Dry Ice) | 97.5189 lb./ft.3 at -109.3° F |
| Density Liquid | 63.69 lb./ft.3 at 0° F |
| Density Gas | 0.1234 lb./ft.3 at 32° F |
Q. Why it is called dry ice?
Dry ice is the solid form of carbon dioxide (CO2). It’s called “dry ice” because it does not melt like wet ice. Instead, dry ice converts into carbon dioxide gas.
Q. What is dry ice samacheer Class 8 used for?
Solid carbon dioxide, called as dry ice is used as a refrigerant. The gas is so cold that moisture in the air condenses on it, creating a dense fog which is used in stage shows and movie effects.
Q. What is adolescence Class 8 samacheer?
Answer: Adolescence is a stage of rapid growth and development.
Q. What is green manure Class 8 samacheer?
Answer: The undesirable plants growing naturally with crop plants are called weeds. These plants gradually decompose and turn into green manure which helps in ensuring the soil in nitrogen and phosphorous. Application of green manure always enhance the growth and yield of the crops.
Q. What is heavier air or nitrogen?
Nitrogen gas is only slightly lighter than air and readily mixes with air at room temperature. Cold vapors are more dense and will settle. Liquid nitrogen, a cryogenic liquid, has a very low boil- ing point of –320°F.
Q. Which air is heavier hot or cold?
Cold air is always heavier than an equal volume of hot air. “Air” is actually a mixture of several gases. By volume, dry air contains 78.09 percent nitrogen, 20.95 percent oxygen, 0.93 percent argon, 0.039 percent carbon dioxide and small amounts of other gases.
Q. Is nitrogen the same as oxygen?
All N2 and O2 which are commercially available are produced form of the air. Nitrogen is colourless, odourless and tasteless gas. Oxygen is also without colour, odour and taste. Compared to nitrogen the oxygen reacts with most of the chemical elements.
Q. Which gas is heavier than air?
While many people think that propane gas is “lighter’ than air and will dissipate into the atmosphere, propane is actually a dense fuel that is 50 percent heavier than atmospheric air at sea level.
Q. Is a fart heavier than air?
The hydrogen and methane contained in much flatulence are both lighter than air. The smellier farts though, are going to have a larger concentration of sulfur-containing compounds, and thus, are going to have higher weights, to the point of the mixture quite possibly being heavier than air on the whole.
Q. What is the heaviest gas on Earth?
radon
Q. Does LPG rise or sink?
In the event of an LPG gas leak, it is important to remember that LPG is heavier than air. Leaking gas will settle to the lowest point, including basements and under houses.
Q. At what temperature does LPG become a gas?
The pressure at which LPG becomes liquid, called its vapour pressure, likewise varies depending on composition and temperature; for example, it is approximately 220 kilopascals (32 psi) for pure butane at 20 °C (68 °F), and approximately 2,200 kilopascals (320 psi) for pure propane at 55 °C (131 °F) (This comparison is …
Q. Is methane lighter than air or heavier?
Methane is lighter than air, colourless and, despite what you might think considering animals burp it out, odourless. Methane (CH4) molecules have four hydrogen atoms and a central carbon atom.
Q. Can an LP gas leak ignite?
LPG concentrations as low as two per cent can ignite the air and result in fire and explosion, while natural gas can ignite with a concentration just over five per cent. If you smell gas, and is it safe to do so, stay calm and: Turn off your gas appliances.
Q. Can a propane leak kill you?
Inhaling a high concentration of natural gas can lead to asphyxia, the symptoms of which include fatigue and chest pain. Asphyxia occurs when your body is deprived of oxygen, and the more carbon monoxide there is present in the air, the less oxygen you’ll be able to inhale, which can potentially kill you.
Q. Can Propane cause carbon monoxide poisoning?
Carbon Monoxide (CO) and Propane. Properly functioning propane appliances will produce what is called an “ideal burn” during combustion and present no danger of Carbon Monoxide poisoning. Carbon Monoxide poisoning can lead to severe injury and even death.
Q. Is LP gas dangerous?
Propane vapor is not toxic, but it is an asphyxiating gas. That means propane will displace the oxygen in your lungs, making it difficult or impossible to breathe if exposed to high concentrations. If you suspect you have inhaled a significant amount of propane, call 911.