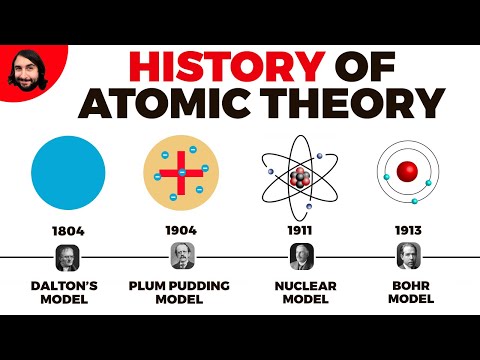
Q. What did Ernest Rutherford contribute to the atomic theory?
Ernest Rutherford is known for his pioneering studies of radioactivity and the atom. He discovered that there are two types of radiation, alpha and beta particles, coming from uranium. He found that the atom consists mostly of empty space, with its mass concentrated in a central positively charged nucleus.
Q. When did Ernest Rutherford contribute to the atomic theory?
1911
Table of Contents
- Q. What did Ernest Rutherford contribute to the atomic theory?
- Q. When did Ernest Rutherford contribute to the atomic theory?
- Q. How did Ernest Rutherford make his discovery about atoms?
- Q. What was Rutherford experiment and what did he discover?
- Q. What are the limitations of Rutherford atomic model?
- Q. Why was a thin foil used?
- Q. Why did Rutherford use gold foil and not magnesium?
- Q. Why did Rutherford use magnesium foil?
- Q. What is the metal foil used for in Rutherford’s experiment?
- Q. Why did Rutherford chose gold?
- Q. What did Rutherford’s alpha particle scattering experiment prove?
- Q. Why do we use alpha particles in Rutherford?
- Q. What does 180 deflection of only very few alpha particles indicate?
- Q. Why was it observed that some of the particles deflected by 180 degrees?
- Q. What causes the alpha particles to deflect backwards?
- Q. Why did some of the alpha particles rebound?
- Q. What would have happened if Rutherford used negative particles?
Q. How did Ernest Rutherford make his discovery about atoms?
Rutherford overturned Thomson’s model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny and heavy nucleus. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure.
Q. What was Rutherford experiment and what did he discover?
Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom.
Q. What are the limitations of Rutherford atomic model?
This atomic model failed to explain stability of atoms. According to the model, electrons revolve around the positively charged nucleus. It’s not possible for a long run as we know atoms are stable while any particle in a circular orbit would undergo acceleration.
Q. Why was a thin foil used?
Why ? It was done to avoid multiple scattering.
Q. Why did Rutherford use gold foil and not magnesium?
The scattering angles would have changed, but the qualitative results would also change: the reason Rutherford chose gold was because it is EXTREMELY malleable. One can stretch gold foil until it is only a few atoms thick in places, which is not possible with aluminum.
Q. Why did Rutherford use magnesium foil?
Rutherford used gold for his scattering experiment because gold is the most malleable metal and he wanted the thinnest layer as possible. The goldsheet used was around 1000 atoms thick. Therefore, Rutherford selected a Gold foil in his alpha scatttering experiment.
Q. What is the metal foil used for in Rutherford’s experiment?
By placing metal foils on top of the uranium, Rutherford showed that part of the ionizing radiation was stopped while another part appeared to pass through the foils. This helped to confirm the work of Becquerel, showing that there were two components of the ionizing radiation.
Q. Why did Rutherford chose gold?
Rutherford’s model of an atom : He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about 1000 atoms thick. α-particles are doubly-charged helium ions. Since they have a mass of 4µ, the fast-moving α-particles have a considerable amount of energy.
Q. What did Rutherford’s alpha particle scattering experiment prove?
Rutherford’s gold foil experiment demonstrated that almost all of the mass of an atom is in a tiny volume in the center of the atom which Rutherford called the nucleus. This positively charged mass was responsible for deflecting alpha particles propelled through the gold foil.
Q. Why do we use alpha particles in Rutherford?
Like all good scientists, Rutherford was curious. He wondered how he could use alpha particles to learn about the structure of the atom. A: The alpha particles would penetrate the gold foil. Alpha particles are positive, so they might be repelled by any areas of positive charge inside the gold atoms.
Q. What does 180 deflection of only very few alpha particles indicate?
Answer. Answer: Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space. According to Rutherford, positive charge is concentrated in the center of the atom, called nucleus.
Q. Why was it observed that some of the particles deflected by 180 degrees?
2.1 Theory According to the plum pudding model, the α particles would barely be deflected as it passed through the foil because the coulomb repulsion is spread out over the positive pudding. Rutherford found instead that a small percentage of α particles were deflected as much as 180 degrees.
Q. What causes the alpha particles to deflect backwards?
Explanation: Alpha particles are are positively charges particles that are made up of 2 protons, 2 neutrons and zero electrons. However, he found that the particles path would be shifted or deflected when passing through the foil. This is due to the fact that like charges repel each other.
Q. Why did some of the alpha particles rebound?
” Only a few particles bounced back because the nucleus is so small / atom is mostly empty space that few collided with a nucleus . If they bounced back it was because of alpha particle and nucleus are positive : repulsion if they bounced back it was because nucleus is massive / dense so alpha particles rebound . “
Q. What would have happened if Rutherford used negative particles?
Both the nucleus and the alpha particle are positive and the alpha is repulsed by the nucleus. If the nucleus was negative then the alpha would be attracted to it. An actual collision would be unlikely but the alpha particles would be deflected towards the nucleus.