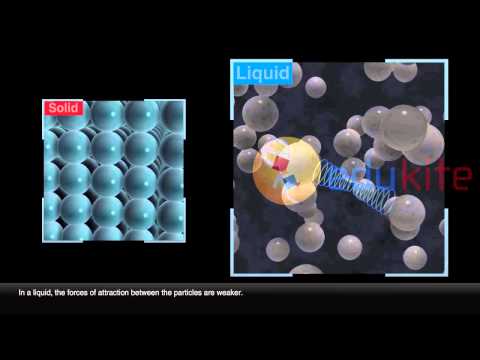
Q. What do the particles in solids liquids and gases have in common?
Gases, liquids and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. gas are well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern.
Q. Is there any similarities in solids liquids and gases?
Solid, Liquids, and Gases. Sold, liquid, and gas all have volume and shape. They are all made up of atoms, molecules, or ions. Liquids and solids can be referred to as condensed phased because their particles are close together. Liquids and gases flow easily because their particles can move or slide past one another.
Table of Contents
- Q. What do the particles in solids liquids and gases have in common?
- Q. Is there any similarities in solids liquids and gases?
- Q. In what way are liquids and gases alike in what way are liquids and solids different?
- Q. Why do solids liquids and gases have different properties?
- Q. How would you make a model to demonstrate movement of particles in solids liquids and gases?
- Q. In what ways are liquids and gases alike in what ways are liquids and solids different?
- Q. What is the same about a particle as gas liquid and solid?
- Q. What happens to the particles of a solid when the solid changes into a gas?
- Q. What are the similarities and differences of the particle motion in solids and liquids?
- Q. In what way are liquids and gases alike?
- Q. How are particles in solids, liquids and gases different?
- Q. How are the particles in a solid packed together?
- Q. How are liquids and gases related and how are they related?
- Q. How are particles in a gas well separated?
Q. In what way are liquids and gases alike in what way are liquids and solids different?
In what way are liquids and gases alike? In what way are liquids and solids different? Liquids and Gasses are alike because both of them take the shape of their containers. Liquids have an indefinite shape while a solid has a definite shape.
Q. Why do solids liquids and gases have different properties?
Solids, liquids and gases are different mainly because of their lattice arrangements and the cohesive forces between the molecules. The cohesive forces between their molecules are also very weak. This gives gases their property to flow and compressibility.
Q. How would you make a model to demonstrate movement of particles in solids liquids and gases?
Take a piece of stretched balloon and 4. Tie it to the mouth of jar tightly with rubber band5. Sew a string to the middle of balloon. Observation: Now the pull the string gently, we can observe the movementOf solidsIf you pull rapidly we can see the movement of solid getting converting to Liquid and gas.
Q. In what ways are liquids and gases alike in what ways are liquids and solids different?
Q. What is the same about a particle as gas liquid and solid?
The particles in a liquid usually are still touching but there are some spaces between them. The gas particles have big distances between them. Solid In a solid, the attractive forces keep the particles together tightly enough so that the particles do not move past each other….Liquids Solids and Gases:
| Definite shape | definite volume | |
|---|---|---|
| gas | no | no |
Q. What happens to the particles of a solid when the solid changes into a gas?
Some substances can change from the solid state to the gas state without ever becoming a liquid. During this process, known as sublimation, the sur- face particles of the solid gain enough energy to become a gas. One example of a substance that undergoes sublimation is dry ice.
Q. What are the similarities and differences of the particle motion in solids and liquids?
Similarities: Solids and liquids both have a definite volume. Moreover, the particles found in them are moving constantly. Differences: Solids have particles that are tightly packed and they form a pattern. In this case, they move and vibrate in place.
Q. In what way are liquids and gases alike?
Liquids and gases are similar in both shape and volume because they both have its shape determined by its surroundings. between particles? Liquids and gases are different in the amount of space between particles. Particles in a liquid are close together, but still have a slight movement.
Q. How are particles in solids, liquids and gases different?
The energy of particles varies between solids, liquids and gases. Subsequently, their different arrangement and movement results in them having different properties. The particles in solids have low energy. There is very strong attraction between particles. Consequently, the particles are tightly packed, and held together in a fixed arrangement.
Q. How are the particles in a solid packed together?
The particles in most solids are closely packed together. Even though the particles are locked into place and cannot move or slide past each other, they still vibrate a tiny bit. Ice is water in its solid form or state. Ice keeps its shape when frozen, even if it is removed from its container.
Q. How are liquids and gases related and how are they related?
gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other. solid vibrate (jiggle) but generally do not move from place to place. Liquids and solids are often referred to as condensed phases because the particles are very close together.
Q. How are particles in a gas well separated?
Note that: Particles in a: gas are well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern. Particles in a: gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other.