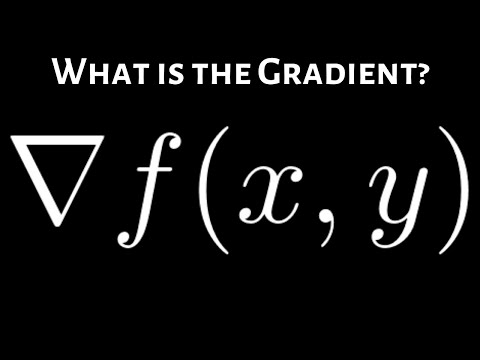Q. What does a shallow gradient mean?
A shallower gradient is the equivalent to a weaker isocratic solvent. As Uwe pointed out, overall, this improves resolution (but suffers from “diminishing returns”). “Overall” may or may not apply in specific cases.
Q. What is steep gradient?
Gradient is a measure of how steep a slope is. The greater the gradient the steeper a slope is. The smaller the gradient the shallower a slope is.
Table of Contents
- Q. What does a shallow gradient mean?
- Q. What is steep gradient?
- Q. What gradient means?
- Q. What does isocratic mean?
- Q. What is difference between isocratic and gradient?
- Q. What is isocratic elution?
- Q. Why is gradient elution better than isocratic?
- Q. Why is gradient elution used?
- Q. How does separation occur in HPLC?
- Q. How do you increase separation in HPLC?
- Q. Is HPLC quantitative or qualitative?
- Q. How do you separate two peaks in HPLC?
- Q. Why do peaks split in HPLC?
- Q. What is Ghost peak in HPLC?
- Q. What is the troubleshooting in HPLC?
- Q. Why does RSD fail in HPLC?
- Q. What causes shouldering in HPLC?
- Q. What causes tailing in HPLC?
- Q. What is fronting in HPLC?
- Q. What is SST in HPLC?
- Q. What is ODS and BDS column?
- Q. What is difference between ODS and C18 column?
- Q. Which column is more polar c8 or C18?
- Q. Which detector is used in HPLC?
- Q. Is C18 column polar or nonpolar?
- Q. How many types of detectors are there in HPLC?
- Q. Which detector Cannot be used in HPLC?
- Q. Which is the most sensitive detector?
- Q. What is rid in HPLC?
Q. What gradient means?
1a : the rate of regular or graded (see grade entry 2 sense transitive 2) ascent or descent : inclination. b : a part sloping upward or downward.
Q. What does isocratic mean?
Filters. (chemistry, of an HPLC system) Which resolves a solute using a solvent system that does not change composition during the run.
Q. What is difference between isocratic and gradient?
Isocratic means that the mixture of your mobile phase is consistent over the complete testing time. Using a gradient implies that the compounding of the eluent mixture is changed during measurement and so influences the retention of analytes. The separation can be either accelerated or decelerated.
Q. What is isocratic elution?
Isocratic elution is a term used in chromatography when the mobile phase has a constant concentration. Here, the concentration of the mobile phase is constant throughout the chromatographic process. Therefore, the peaks elute in the same order.
Q. Why is gradient elution better than isocratic?
Use of gradient elution has several advantages over isocratic elution: (i) it allows the separation of components in the sample that have significantly different affinities toward the surface (e.g., weakly binding proteins eluting early and strongly bind proteins late in the gradient) and (ii) it alleviates the need …
Q. Why is gradient elution used?
Changes in solvent strength are accompanied by a simultaneous change in selectivity for many compounds. Thus, gradient elution provides an effective means of selectivity optimization for samples with a wide retention range in a reasonable separation time with sharper peaks for all sample components.
Q. How does separation occur in HPLC?
The components of a mixture are separated from each other due to their different degrees of interaction with the absorbent particles. This causes different elution rates for the different components and leads to the separation of the components as they flow out the column.
Q. How do you increase separation in HPLC?
Depending on the situation, separations can sometimes be improved by increasing the column plate number, by using smaller particles or by increasing column length. The disadvantages of these approaches are higher operating pressures and increased separation times for longer columns.
Q. Is HPLC quantitative or qualitative?
Two situations exist for qualitative analysis in HPLC & GC: The sample components are known and peaks need to be assigned. By injecting standards of the pure compound assign the peaks in the chromatogram based on the retention time of the standard.
Q. How do you separate two peaks in HPLC?
My hunch is that a change in the proportion of acetonitrile in the mobile phase may help to separate the peaks. You could also try reducing the flow rate of the mobile phase, and reducing the column temperature.
Q. Why do peaks split in HPLC?
The most common causes for peak splitting are i) too strong injection solvent compared to mobile phase composition at elution, ii) column channelling, iii) partially clogged part of the system (column, filter etc.). A too high acquisition rate may also cause jagged peaks.
Q. What is Ghost peak in HPLC?
Ghost peaks are contaminant peaks that appear even when no sample is injected. There are many causes for ghost peaks and this note will describe how to troubleshoot these contaminant peaks, when you see them. The primary cause of a ghost peak is a dirty pre-column or column.
Q. What is the troubleshooting in HPLC?
Pumping system problems are usually easy to spot and correct. Some of the more common symptoms are erratic retention times, noisy baselines, or spikes in the chromatogram. Leaks at pump fittings or seals will result in poor chromatography. Buffer salts should be flushed from the system daily with fresh deionized water.
Q. Why does RSD fail in HPLC?
Re: RSD failing HPLC If the RDS of the ratio is substantially worse than that of the individual peaks, that suggests that the major contributions to error are uncorrelated between the peaks, so look at thinks like peak shape, integration settings, baseline noise, etc.
Q. What causes shouldering in HPLC?
Blocked frit may cause tailing or it may cause split peak. A blocked frit can cause the fraction of the sample to spread on the surface of the column faster and the part of the sample is delayed and this causes Peak Splitting. 1) HPLC analysis with column reversal to improve the peak shape.
Q. What causes tailing in HPLC?
The primary cause of peak tailing is the occurrence of more than one mechanism of analyte retention. Secondary analyte interactions, with ionised silanols on the silica surface, give rise to peak tailing. These interactions need to be minimised to achieve acceptable peak shapes.
Q. What is fronting in HPLC?
Peaks fronting occurs when the sample capacity of the analytical column is exceeded, which can happen in both GC and HPLC experiments. This overloading effect results from poor sample solubility in the stationary phase, the injection of too much sample, or operating at a “k” value (capacity factor) that is too low.
Q. What is SST in HPLC?
System suitability test (SST) is a test to determine the suitability and effectiveness of chromatographic system prior to use. These mixtures are used to establish characteristic chromatographic parameters, such as the number of effective theoretical plates, resolution, asymmetry, detection limit and selectivity.
Q. What is ODS and BDS column?
ODS and BDS are two columns used for reverse-phase chromatography. The key difference between ODS and BDS column is that ODS column contains free –OH functional groups, whereas BDS column contains deactivated –OH groups. Moreover, ODS columns have high peak tailing while BDS columns are designed to reduce peak tailing.
Q. What is difference between ODS and C18 column?
The AQ type C18 column, such the ODS-B, has an end-capping that reduces phase collapse greatly, so it can be run in 100% water if needed. The ODS-A column has a more typical hydrophobic end-capping. Compounds that require more than 50% organic to elute will be less affected by the hydrophilic end-capping on the ODS-B.
Q. Which column is more polar c8 or C18?
C18 has 18 carbon atoms while C8 has only 8 carbon atoms. C18 has a longer carbon chain, but C8 has a shorter one. C18 has higher retention while C8 has shorter retention. C18 has higher hydrophobicity, but C8 has a lower hydrophobicity….Follow Pharmaguideline.
| Like | Follow |
|---|---|
| Follow | Install |
| Join |
Q. Which detector is used in HPLC?
HPLC Detectors
- UV-Vis Detectors. The SPD-20A and SPD-20AV are general-purpose UV-Vis detectors offering an exceptional level of sensitivity and stability.
- Refractive Index Detector.
- Fluorescence Detectors.
- Evaporative Light Scattering Detector.
- Conductivity Detector.
Q. Is C18 column polar or nonpolar?
A C18 column is an example of a “reverse phase” column. Reverse phase columns are often used with more polar solvents such as water, methanol or acetonitrile. The stationary phase is a nonpolar hydrocarbon, whereas the mobile phase is a polar liquid.
Q. How many types of detectors are there in HPLC?
They are of three types, i.e. fixed wavelength detectors, variable wavelength detectors and the diode array detectors.
Q. Which detector Cannot be used in HPLC?
A UV detector cannot be used with solvent which has UV absorbance. Sometimes the organic solvent used for GPC analysis absorbs UV, and thus UV detector cannot be used. It provides a direct relationship between the intensity and analyte concentration.
Q. Which is the most sensitive detector?
Electron capture detector
Q. What is rid in HPLC?
A differential refractometer (DRI), or refractive index detector (RI or RID) is a detector that measures the refractive index of an analyte relative to the solvent. They are often used as detectors for high-performance liquid chromatography and size exclusion chromatography.