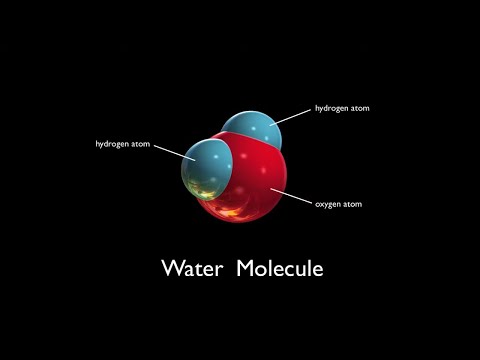
Q. What does a water molecule contain?
A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds.
Q. What holds molecules of water together?
Strong linkages—called covalent bonds—hold together the hydrogen (white) and oxygen (red) atoms of individual H2O molecules. Covalent bonds occur when two atoms—in this case oxygen and hydrogen—share electrons with each other. Each H2O can bind to a maximum of four neighbors through these so-called hydrogen bonds.
Table of Contents
- Q. What does a water molecule contain?
- Q. What holds molecules of water together?
- Q. Is oxygen negative or positive in water?
- Q. Is water more positive or negative?
- Q. How does hydrogen bond with oxygen?
- Q. Which is the strongest hydrogen bond?
- Q. What is the strongest evidence for hydrogen bonding?
- Q. What is the strongest intermolecular force in hi?
- Q. What is the weakest intermolecular force?
- Q. Is a hydrogen bond?
- Q. How do you know if a molecule can hydrogen bond?
- Q. What forms a hydrogen bond?
- Q. Are hydrogen bonds strong in DNA?
- Q. Is HF a hydrogen bond?
- Q. What is the difference between a covalent bond and a hydrogen bond?
- Q. Are covalent or ionic bonds stronger?
- Q. Which is stronger hydrogen or covalent bonds How do you know?
- Q. Why is hydrogen a covalent bond?
- Q. Is HCN a covalent bond?
- Q. Is CaO a covalent bond?
- Q. Does HCN have a covalent bond?
- Q. Is C6H12O6 a covalent bond?
- Q. What is the polarity of hydrogen cyanide?
- Q. How many bonding pairs are in hydrogen cyanide?
- Q. Why is C in the middle of HCN?
- Q. Does CO2 have a triple bond?
- Q. How many bonds are in CO2?
- Q. Is CO2 bonding or antibonding?
Q. Is oxygen negative or positive in water?
Since the oxygen end of a water molecule is slightly negative and the hydrogen end is slightly positive, it makes sense that water molecules attract one another.
Q. Is water more positive or negative?
There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Because of these charges, the slightly positive hydrogen atoms repel each other and form the unique shape seen in [link].
Q. How does hydrogen bond with oxygen?
The hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons, making for a very polar bond. The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule (see Figure below ).
Q. Which is the strongest hydrogen bond?
HF
Q. What is the strongest evidence for hydrogen bonding?
The boiling points of NH3, H2O, and HF are abnormally high compared with the rest of the hydrides in their respective periods.” is the strongest evidence for hydrogen bonding.
Q. What is the strongest intermolecular force in hi?
IMF
Q. What is the weakest intermolecular force?
The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction.
Q. Is a hydrogen bond?
Hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; such a bond is weaker than an ionic bond or covalent bond but stronger than van der Waals forces.
Q. How do you know if a molecule can hydrogen bond?
Any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Hydrogen bonds also occur when hydrogen is bonded to fluorine, but the HF group does not appear in other molecules.
Q. What forms a hydrogen bond?
Hydrogen bonding is a special type of dipole-dipole attraction between molecules, not a covalent bond to a hydrogen atom. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
Q. Are hydrogen bonds strong in DNA?
Hydrogen bonds are weak, noncovalent interactions, but the large number of hydrogen bonds between complementary base pairs in a DNA double helix combine to provide great stability for the structure.
Q. Is HF a hydrogen bond?
Although a diatomic molecule, HF forms relatively strong intermolecular hydrogen bonds. Solid HF consists of zig-zag chains of HF molecules. The HF molecules, with a short H–F bond of 95 pm, are linked to neighboring molecules by intermolecular H–F distances of 155 pm.
Q. What is the difference between a covalent bond and a hydrogen bond?
Covalent bond is a primary chemical bond formed by the sharing of electron pairs. Covalent bonds are strong bonds with greater bond energy. Hydrogen bond is a weak electrostatic attraction between the hydrogen and an electronegative atom due to their difference in electronegativity.
Q. Are covalent or ionic bonds stronger?
Ionic bonds are stronger. It takes more energy to pull the two atoms apart to infinity than it does in a covalent bond.
Q. Which is stronger hydrogen or covalent bonds How do you know?
Covalent Bonds are stronger than hydrogen bonds because a covalent bond is an attraction within molecules whereas hydrogen bonds are attractions between molecules and are therefore generally weaker.
Q. Why is hydrogen a covalent bond?
The hydrogen molecule is the simplest substance having a covalent bond. It forms from two hydrogen atoms, each with one electron in a 1s orbital. Both hydrogen atoms share the two electrons in the covalent bond, and each acquires a helium-like electron configuration.
Q. Is HCN a covalent bond?
The formula HCN shows that hydrogen cyanide has a single covalent bond between the hydrogen and carbon atoms and a triple covalent bond between the carbon and nitrogen atoms.
Q. Is CaO a covalent bond?
Decide if the following formulas represent Ionic or Covalent compounds….Ionic or Covalent.
| A | B |
|---|---|
| CO2 | Covalent |
| NH3 | Covalent |
| MgBr2 | Ionic |
| CaO | Ionic |
Q. Does HCN have a covalent bond?
There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, : , each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, ::: , representing three pairs of shared electrons, and a lone pair of electrons …
Q. Is C6H12O6 a covalent bond?
The difference is EN of C, H and O aren’t great enough to have one atom give up its electrons, so a true ionic bond does not form. This makes all the bonds in C6H12O6 covalent bonds.
Q. What is the polarity of hydrogen cyanide?
HCN is a polar molecule because of the large electronegative difference between Nitrogen(3.04) and hydrogen(2.2) due to which the linear-shaped molecule has unequal sharing of charge and results in non zero dipole moment making the molecule polar. HCN is acidic in nature.
Q. How many bonding pairs are in hydrogen cyanide?
4 bonds
Q. Why is C in the middle of HCN?
Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. As Carbon is the least electronegative atom in this molecule, it will take the central position. …
Q. Does CO2 have a triple bond?
The initial VSEPR shape for the CO2 molecule is Tetrahedral. For each multiple bond (double/triple bond), subtract one electron from the final total. The CO2 molecule has 2 double bonds so minus 2 electrons from the final total.
Q. How many bonds are in CO2?
Q. Is CO2 bonding or antibonding?
Bonding Order = number of bonding electrons – number of antibonding electrons/2. So for CO2, there is a total of 16 electrons, 8 of which are antibonding electrons. So 16 – 8 = 8; divided by 2 = 4. So, 4 is the bonding order of CO2.