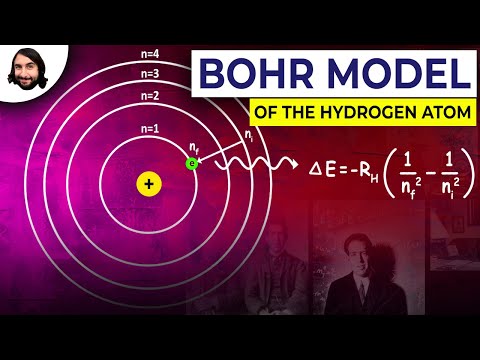
Q. What does K stand for in the Bohr model?
The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Figure 2: Bohr diagrams for neutral lithium, fluorine and aluminum atoms. Each shell can only hold certain number of electrons. K shell can have 2, L can have 8 , M can have 18 electrons and so on.
Q. How is an atom in the Bohr model charged?
In atomic physics, the Bohr model depicts an atom as a small, positively charged nucleus surrounded by electrons. These electrons travel in circular orbits around the nucleus—similar in structure to the solar system, except electrostatic forces rather than gravity provide attraction.
Table of Contents
- Q. What does K stand for in the Bohr model?
- Q. How is an atom in the Bohr model charged?
- Q. What element is represented in this Bohr model?
- Q. What are the issues with the Bohr model?
- Q. What are two problems with the Bohr model?
- Q. Why is the Bohr model useful?
- Q. Why was Bohr rejected?
- Q. Is the Bohr model accurate?
- Q. What is the difference between Bohr model and quantum model?
- Q. Why was Rutherford’s model accepted?
- Q. Did Rutherford actually see the atomic nucleus?
- Q. What would have happened if the plum pudding model was correct?
- Q. Why was Thomson’s model replaced by Rutherford’s model?
- Q. Why was Thomson’s atomic model discarded?
Q. What element is represented in this Bohr model?
Circulating round the nucleus are the electrons in various orbits of different energy levels. Electrons are negatively charged and represented by the symbol ‘e’. In the given image the number of protons are -6. Hence the element in question is Carbon as Carbon has the atomic number 6.
Q. What are the issues with the Bohr model?
The main problem with Bohr’s model is that it works very well for atoms with only one electron, like H or He+, but not at all for multi-electron atoms.
Q. What are two problems with the Bohr model?
The Bohr Model considers electrons to have both a known radius and orbit, which is impossible according to Heisenberg. The Bohr Model is very limited in terms of size. Poor spectral predictions are obtained when larger atoms are in question. It cannot predict the relative intensities of spectral lines.
Q. Why is the Bohr model useful?
The Bohr model is important because it was the first model to postulate the quantization of electron orbits in atoms. Thus, it represents an early quantum theory that gave a start to developing modern quantum theory. It introduced the concept of a quantum number to describe atomic states.
Q. Why was Bohr rejected?
Bohr’s model failed because it treated electrons according to the laws of classical physics. Unfortunately, those laws only apply to fairly large objects. Back when Bohr was developing his model, scientists were only beginning to realize that the laws of classical physics didn’t apply to matter as tiny as the electron.
Q. Is the Bohr model accurate?
This model was proposed by Niels Bohr in 1915; it is not completely correct, but it has many features that are approximately correct and it is sufficient for much of our discussion.
Q. What is the difference between Bohr model and quantum model?
The Bohr model and quantum model are models that explain the structure of an atom. The key difference between Bohr and quantum model is that Bohr model states that electrons behave as particles whereas quantum model explains that the electron has both particle and wave behavior.
Q. Why was Rutherford’s model accepted?
Because only very few of the alpha particles in his beam were scattered by large angles after striking the gold foil while most passed completely through, Rutherford knew that the gold atom’s mass must be concentrated in a tiny dense nucleus.
Q. Did Rutherford actually see the atomic nucleus?
Though Rutherford still didn’t know what was in this nucleus he had discovered (protons and neutrons would be identified later), his insight in 1911, which overturned the prevailing plum pudding model of the atom, had opened the way for modern nuclear physics.
Q. What would have happened if the plum pudding model was correct?
If the plum pudding model was correct, all of the alpha particles would have passed straight through the foil with little or no deflection. Alpha particles were known to be much, much more dense than gold.
Q. Why was Thomson’s model replaced by Rutherford’s model?
In 1911, Rutherford showed that Thomson’s model was “wrong”: the distribution of positive and negative particles was not uniform. Rutherford showed that the atom contains a small, massive, positively charged nucleus. He also agreed with Nagaoka that the electrons move in circular orbits outside the nucleus.
Q. Why was Thomson’s atomic model discarded?
Answer. Thomson’s atomic model was discarded because it could not explain the stability of an atom. Also , it could not explain the results of ionisation and scattering experiment carried out by Rutherford.