The frequency factor is A , also known as the pre-exponential factor. This is essentially an experimentally-acquired constant. It generally represents the frequency of collisions between molecules in a reaction. It is the fraction of molecules that would react if there existed no energy barrier.
Q. Which factor in the Arrhenius equation represents the frequency of collisions and the probability?
pre-exponential factor
Table of Contents
- Q. Which factor in the Arrhenius equation represents the frequency of collisions and the probability?
- Q. What is the frequency factor in Arrhenius equation?
- Q. What does frequency factor depend on?
- Q. Does frequency factor affect rate constant?
- Q. What is the value of frequency factor?
- Q. How do u find the frequency?
- Q. Does Catalyst affect equilibrium constant?
- Q. Does a catalyst increase the rate constant?
- Q. Does a catalyst change the rate?
- Q. Does a catalyst decrease the rate constant?
- Q. What can change the rate constant?
- Q. Does pressure change rate constant?
- Q. Why does temperature increase rate constant?
- Q. Does rate constant depend on temperature?
- Q. Does rate of reaction depend on temperature?
- Q. Does equilibrium constant depend on temperature?
- Q. What does the equilibrium constant depend on?
- Q. What is the temperature of equilibrium constant?
- Q. How many equilibrium positions are there?
- Q. How do I calculate delta G?
- Q. Is exothermic reaction hot or cold?
- Q. How can you increase the yield of an exothermic reaction?
- Q. How do you speed up an exothermic reaction?
- Q. What decreases in an exothermic reaction?
- Q. What four factors would allow for the fastest reaction?
Q. What is the frequency factor in Arrhenius equation?
The frequency factor, A, is approximately constant for such a small temperature change. This problem concerns the quantity e-(EA / RT), the fraction of molecules with energies equal to or in excess of the activation energy. Let’s assume an activation energy of 50 kJ mol-1, or, equivalently, J mol-1.
Q. What does frequency factor depend on?
According to collision theory, the frequency factor, A, depends on how often molecules collide when all concentrations are 1 mol/L and on whether the molecules are properly oriented when they collide. Values of A for some reactions can be found at Collision theory#Steric factor.
Q. Does frequency factor affect rate constant?
You can use the Arrhenius equation to show the effect of a change of temperature on the rate constant – and therefore on the rate of the reaction. The frequency factor, A, in the equation is approximately constant for such a small temperature change.
Q. What is the value of frequency factor?
The frequency factor is used to describe the rate of molecular collisions that occur in the chemical reaction. You can use it to measure the frequency of the molecular collisions that have the proper orientation between particles and appropriate temperature so that the reaction can occur.
Q. How do u find the frequency?
How to calculate frequency
- Determine the action. Decide what action you want to use to determine the frequency.
- Select the length of time. Select the length of time over which you will measure the frequency.
- Divide the numbers. To calculate frequency, divide the number of times the event occurs by the length of time.
Q. Does Catalyst affect equilibrium constant?
Equilibrium constants are not changed if you add (or change) a catalyst. The only thing that changes an equilibrium constant is a change of temperature. The position of equilibrium is not changed if you add (or change) a catalyst. A catalyst speeds up both the forward and back reactions by exactly the same amount.
Q. Does a catalyst increase the rate constant?
The addition of a catalyst lowers the activation energy of a reaction. This means that the rate constant will increase, as the activation energy is a term used to calculate this value.
Q. Does a catalyst change the rate?
Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
Q. Does a catalyst decrease the rate constant?
An catalyst will lower the activation energy of a reaction. Remember from the Arrhenius expression that the rate constant depends on the activation energy, among other variables. A catalyst will affect both the rate and the rate constant for a given reaction.
Q. What can change the rate constant?
These are all included in the so-called rate constant (k)- which is only actually constant if all you are changing is the concentration of the reactants. If you change the temperature or the catalyst, for example, the rate constant changes. This is shown mathematically in the Arrhenius equation.
Q. Does pressure change rate constant?
Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Changing the pressure on a reaction which involves only solids or liquids has no effect on the rate.
Q. Why does temperature increase rate constant?
Going back to the rate law equation, it follows that a higher rate constant results in a higher reaction rate. This makes sense because as temperature increases, molecules move faster and collide more frequently, resulting in an increased fraction of molecules with higher energy than the activation energy.
Q. Does rate constant depend on temperature?
An increase in temperature increases the rate constant and hence the rate. An increase in concentration increases the rate but not the rate constant. Temperature affects k and k affects R so, temperature affects both while concentration affects only Rate of reactions.
Q. Does rate of reaction depend on temperature?
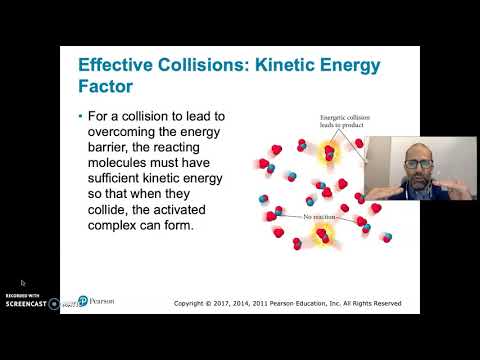
An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision (Figure.
Q. Does equilibrium constant depend on temperature?
It does, however, depend on the temperature of the reaction. This is because equilibrium is defined as a condition resulting from the rates of forward and reverse reactions being equal. If the temperature changes, the corresponding change in those reaction rates will alter the equilibrium constant.
Q. What does the equilibrium constant depend on?
Equilibrium constant depends on temperature and is independent of the actual quantities of reactants and products, the presence of a catalyst and the presence of inert material. It is also independent of concentrations, pressures and volumes of reactants and products.
Q. What is the temperature of equilibrium constant?
This is typical of what happens with any equilibrium where the forward reaction is exothermic. Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the temperature increases the value of the equilibrium constant….
| temperature | Kp |
|---|---|
| 700 K | 54 |
Q. How many equilibrium positions are there?
There are infinitely many different equilibrium positions that satisfy K. We could have [B]=20 M and [A]=0.1 M. Or we could have [B]=1 M and [A]=0.005 M. Those are two different equilibrium positions that are both at equilibrium.
Q. How do I calculate delta G?
the delta G equation, combines the enthalpy vs. entropy relation….Gibbs free energy calculator
- ΔG = ΔH − T * ΔS ;
- ΔH = ΔG + T * ΔS ; and.
- ΔS = (ΔH − ΔG) / T .
Q. Is exothermic reaction hot or cold?
Lesson Summary When a chemical reaction combines two or more things and makes a chemical bond, energy is released, so it is an exothermic reaction. These reactions usually feel hot because heat is given off. If a reaction breaks one or more bonds, energy is needed, or consumed, so it is an endothermic reaction.
Q. How can you increase the yield of an exothermic reaction?
If the forward reaction that forms the product is exothermic, then a decrease in temperature will favour this reaction and the product yield will increase. Increasing the temperature will decrease the product yield. Increasing the pressure favours the side of the equilibrium with the least number of gas molecules.
Q. How do you speed up an exothermic reaction?
Hi. As a general rule, increasing the temperature will increase the reaction rate (for exothermic and endothermic) reactions simply because it means more energy available in the system.
Q. What decreases in an exothermic reaction?
An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. A system that releases heat to the surroundings, an exothermic reaction, has a negative ΔH by convention, because the enthalpy of the products is lower than the enthalpy of the reactants of the system.
Q. What four factors would allow for the fastest reaction?
Reactant concentration, the physical state of the reactants, and surface area, temperature, and the presence of a catalyst are the four main factors that affect reaction rate.