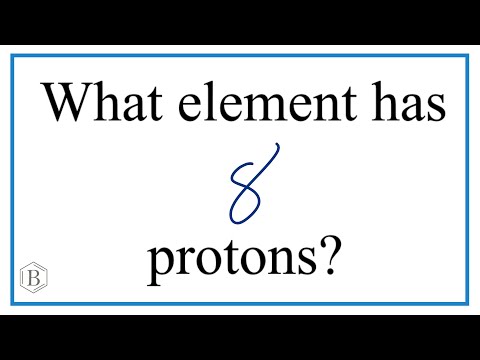
Q. What element has an atomic number of 8 and 8 neutrons?
oxygen atom
Q. What is the mass number and name of an element with 8 protons and 8 neutrons *?
| Name | Oxygen |
|---|---|
| Atomic Mass | 15.999 atomic mass units |
| Number of Protons | 8 |
| Number of Neutrons | 8 |
| Number of Electrons | 8 |
Q. What element has an atomic mass of 8?
Oxygen
Table of Contents
- Q. What element has an atomic number of 8 and 8 neutrons?
- Q. What is the mass number and name of an element with 8 protons and 8 neutrons *?
- Q. What element has an atomic mass of 8?
- Q. What element is a gas with 8 protons and 8 neutrons?
- Q. What element has 8 protons and 9 Neutrons?
- Q. What symbol has 9 Neutrons?
- Q. Can oxygen have 9 electrons?
- Q. What has 9 protons and 10 neutrons?
- Q. What is the mass number of 19 9 F?
- Q. Why does fluorine have 10 neutrons?
- Q. What is the number at the end of an isotope’s name?
- Q. What is the difference between average atomic mass and mass number?
- Q. Which element has the least amount of protons?
- Q. What has 53 protons and 74 neutrons?
- Q. Which element has the most number of neutrons?
- Q. Where is uranium found in nature?
- Q. What are 3 uses for uranium?
- Q. Why is uranium-235 so dangerous?
- Q. What is uranium used in today?
- Q. What happens if you eat uranium?
- Q. How much uranium can you legally own?
- Q. How much plutonium will kill you?
Q. What element is a gas with 8 protons and 8 neutrons?
OXYGEN – I
Q. What element has 8 protons and 9 Neutrons?
oxygen
Q. What symbol has 9 Neutrons?
Fluorine – F
Q. Can oxygen have 9 electrons?
Answer 9: Oxygen is atomic number 8 on the periodic table, which means it has 8 protons! This means that a neutral oxygen atom will have 8 electrons (but this number can change!
Q. What has 9 protons and 10 neutrons?
fluorine atoms
Q. What is the mass number of 19 9 F?
| ChEBI Name | fluorine-19 atom |
|---|---|
| Definition | The stable isotope of fluorine with relative atomic mass 18.998403 and nuclear spin 1/2. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
| Supplier Information | |
| Download | Molfile XML SDF |
Q. Why does fluorine have 10 neutrons?
Because in each fluorine nucleus, there are necessarily 9 protons , why necessarily?, there must be 10 neutrons, neutral massive particles, in the fluorine nucleus. Note that 19F is 100% spin active (I=1/2) , it can be regularly used in NMR spectroscopy .
Q. What is the number at the end of an isotope’s name?
mass number
Q. What is the difference between average atomic mass and mass number?
Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that element’s isotopes. The mass number is a count of the total number of protons and neutrons in an atom’s nucleus.
Q. Which element has the least amount of protons?
Atomic Number
| Name | Protons | Neutrons |
|---|---|---|
| Hydrogen | 1 | 0 |
| Helium | 2 | 2 |
| Lithium | 3 | 4 |
| Beryllium | 4 | 5 |
Q. What has 53 protons and 74 neutrons?
iodine
Q. Which element has the most number of neutrons?
Uranium
Q. Where is uranium found in nature?
Uranium occurs in most rocks in concentrations of 2 to 4 parts per million and is as common in the Earth’s crust as tin, tungsten and molybdenum. Uranium occurs in seawater, and can be recovered from the oceans. Uranium was discovered in 1789 by Martin Klaproth, a German chemist, in the mineral called pitchblende.
Q. What are 3 uses for uranium?
Uranium is also used by the military to power nuclear submarines and in nuclear weapons. Depleted uranium is uranium that has much less uranium-235 than natural uranium. It is considerably less radioactive than natural uranium. It is a dense metal that can be used as ballast for ships and counterweights for aircraft.
Q. Why is uranium-235 so dangerous?
Inhaling large concentrations of uranium can cause lung cancer from the exposure to alpha particles. Uranium is also a toxic chemical, meaning that ingestion of uranium can cause kidney damage from its chemical properties much sooner than its radioactive properties would cause cancers of the bone or liver.
Q. What is uranium used in today?
The main use for uranium today is for fuel in nuclear power plants. Depleted uranium (DU) is used in bullets and larger projectiles to make them hard and dense enough to punch through armored targets. It is also used to improve the metal armor used on tanks and other armored vehicles.
Q. What happens if you eat uranium?
Kidney damage has been seen in humans and animals after inhaling or ingesting uranium compounds. Ingesting water-soluble uranium compounds will result in kidney effects at lower doses than following exposure to insoluble uranium compounds. Inhaled insoluble uranium compounds can also damage the respiratory tract.
Q. How much uranium can you legally own?
There is no legal limit on the amount of uranium ore you can own. Once it has been refined, it becomes more problematic. Yellow cake uranium (uranium leachate) is moderately radioactive so should be handled by experts, but there doesn’t seem to be a law against ownership.
Q. How much plutonium will kill you?
You can support Foreign Policy by becoming a subscriber. 5 grams of plutonium to die immediately, compared to about . 1 grams of cyanide. The plutonium at Fukushima isn’t in the air, but inhaling about 20 milligrams of plutonium would probably kill you within a few months. External exposure carries almost no risk.