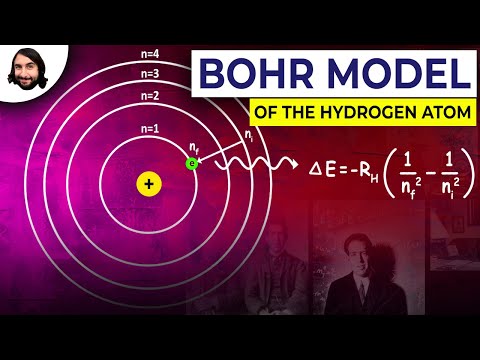
Q. What element is this Bohr model of?
hydrogen atom
The Bohr atomThe Rutherford–Bohr model of the hydrogen atom. In this view, electron orbits around the nucleus resemble that of planets around the sun in the solar system.
Q. What has 47 protons 60 neutrons and 46 electrons?
Silver (Ag) is a silver metal that has the atomic number 47 in the periodic table.
Table of Contents
- Q. What element is this Bohr model of?
- Q. What has 47 protons 60 neutrons and 46 electrons?
- Q. What is the atomic model for silver?
- Q. What is the electron configuration for silver?
- Q. What does 47 mean for silver?
- Q. What is silver composed of?
- Q. What is silver used for?
- Q. What is the shorthand notation for silver?
- Q. Why is silver an exception to electron configuration?
- Q. What are some disadvantages of the Bohr model?
- Q. What does Bohr model stand for?
- Q. What does a Bohr model represent?
- Q. What is the purpose of a Bohr model?
Q. What is the atomic model for silver?
The nucleus consists of 47 protons (red) and 61 neutrons (orange). 47 electrons (white) successively occupy available electron shells (rings). Silver is a transition metal in group 11, period 5, and the d-block of the periodic table. It has a melting point of 962 degrees Celsius.
Q. What is the electron configuration for silver?
[Kr] 4d¹⁰ 5s¹
Silver/Electron configuration
Q. What does 47 mean for silver?
It is classified as a transitional metal. Silver atoms have 47 electrons and 47 protons with 60 neutrons in the most abundant isotope. Characteristics and Properties. Under standard conditions silver is a soft metal that has a shiny metallic finish.
Q. What is silver composed of?
The metal is found in the Earth’s crust in the pure, free elemental form (“native silver”), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining….Silver.
| Hydrogen | Potassium |
|---|---|
| Calcium | |
| Scandium | |
| Titanium | |
| Vanadium |
Q. What is silver used for?
It is used for jewellery and silver tableware, where appearance is important. Silver is used to make mirrors, as it is the best reflector of visible light known, although it does tarnish with time. It is also used in dental alloys, solder and brazing alloys, electrical contacts and batteries.
Q. What is the shorthand notation for silver?
Explanation: For silver , Z=47 . It is thus 11 protons removed from the last Noble Gas, which is krypton , Z=36 . And thus the electronic configuration of silver metal is: [Kr]4d105s1 .
Q. Why is silver an exception to electron configuration?
Now, you have to be a little careful with silver because it is a transition metal, which implies that the occupied d-orbitals are actually lower in energy than the s-orbitals that belong to the highest energy level. The thing to remember here is that in silver’s case, the 4d orbitals will be completely filled.
Q. What are some disadvantages of the Bohr model?
Drawbacks of Bohr’s Theory: The theory could not account for the spectra of atoms more complex than hydrogen. The theory does not give any information regarding the distribution and arrangement of electrons in an atom. It doesn’t explain, the experimentally observed variation in the intensity of the spectral lines of the element.
Q. What does Bohr model stand for?
Bohr model. In atomic physics, the Rutherford-Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus-similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity.
Q. What does a Bohr model represent?
The Bohr Model is a representation of the atom where negatively-charged electrons orbit around a positively-charged nucleus in the same way that the planets of the solar system orbit the sun. In the latter, the force holding the planets and the sun together is a gravitational force; whereas in the Bohr Model of the Atom,…
Q. What is the purpose of a Bohr model?
Bohr’s model of the atom was important because it introduced quantized energy states for the electrons. However, as a model it was only useful for predicting the behavior of atoms with a single electron (H, He +, and Li 2+ ions). Thus, a different model of the atom eventually replaced Bohr’s model.