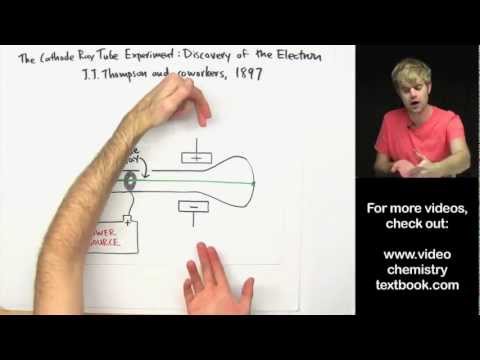
Q. What experiments did JJ Thomson do for the atomic theory?
In 1897, J.J. Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. He demonstrated that cathode rays were negatively charged. In addition, he also studied positively charged particles in neon gas.
Q. What was JJ Thomson’s discovery?
On April 30, 1897, British physicist J.J. Thomson announced his discovery that atoms were made up of smaller components. This finding revolutionized the way scientists thought about the atom and had major ramifications for the field of physics.
Table of Contents
- Q. What experiments did JJ Thomson do for the atomic theory?
- Q. What was JJ Thomson’s discovery?
- Q. What 3 things did JJ Thomson discover?
- Q. How was Thomson’s model different from Dalton’s model of the atom?
- Q. What is JJ Thomson best known for?
- Q. When was J.J. Thomson atomic theory?
- Q. Where did J.J. Thomson discover the electron?
- Q. When was JJ Thomson atomic theory?
- Q. Why is the work of JJ Thomson important?
- Q. What was Thomson’s atomic model?
- Q. What did Thomson claim about the body of an atom?
- Q. How is the Thomson atomic model similar to a plum pudding?
- Q. When did j.j.thomson discover the electron?
Q. What 3 things did JJ Thomson discover?
J. J. Thomson
- Beginnings: School and University.
- Early Research Work.
- Discovery of the Electron – The first subatomic particle.
- The Atom as a Plum Pudding.
- Invention of the Mass Spectrometer.
- Every Hydrogen Atom has only one Electron.
- Discovery of Isotopes of Stable Elements.
- Some Personal Details and the End.
Q. How was Thomson’s model different from Dalton’s model of the atom?
Note: The basic difference between the two models lies in the fact that Dalton proposed that an atom was indivisible and indestructible whereas Thomson worked on the existence of subatomic particles inside an atom and their arrangements i.e., he considered an atom to be a divisible quantity unlike Dalton.
Q. What is JJ Thomson best known for?
Thomson, in full Sir Joseph John Thomson, (born December 18, 1856, Cheetham Hill, near Manchester, England—died August 30, 1940, Cambridge, Cambridgeshire), English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897).
Q. When was J.J. Thomson atomic theory?
In 1904, Thomson proposed a model of the atom as a sphere of positive matter with electrons positioned based on electrostatic forces. So, he not only discovered the electron but determined it was a fundamental part of an atom.
Q. Where did J.J. Thomson discover the electron?
J.J. Thomson attended Trinity College at Cambridge, where he would come to head the Cavendish Laboratory. His research in cathode rays led to the discovery of the electron, and he pursued further innovations in atomic structure exploration.
Q. When was JJ Thomson atomic theory?
Q. Why is the work of JJ Thomson important?
In 1897 Thomson discovered the electron and then went on to propose a model for the structure of the atom. His work also led to the invention of the mass spectrograph.
Q. What was Thomson’s atomic model?
Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom.
Q. What did Thomson claim about the body of an atom?
Thomson’s Atomic model. In 1897, Thomson claimed the basic body of an atom is a sphere that contains electrons (tiny particles within an atom that create a negative charge) and a positively charged “jelly” around the electrons that neutralize the charge of the electrons.
Q. How is the Thomson atomic model similar to a plum pudding?
Thomson’s model of an atom is similar to a plum pudding. Postulate 1: An atom consists of a positively charged sphere with electrons embedded in it. Postulate 2: An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude. Thomson atomic model is compared to watermelon.
Q. When did j.j.thomson discover the electron?
This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier. During cathode ray tube experiment, a negatively charged particle was discovered by J.J. Thomson. This experiment took place in the year 1897.