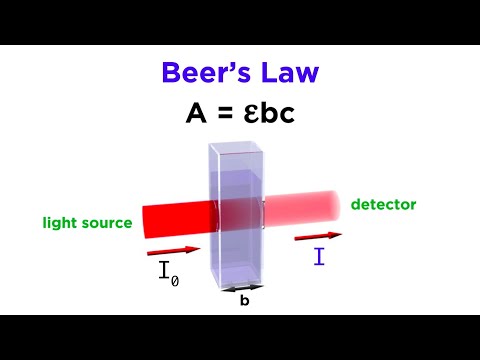
The two main factors that affect absorbance are concentration of the substance and path length. Relation between concentration and absorbance: Absorbance is directly proportional to the concentration of the substance. The higher the concentration, the higher its absorbance.
Q. What is the purpose of measuring the absorbance of a solution at different wavelengths?
In the lab, they are used to determine how much light is absorbed by a colored chemical dissolved in solution. This allows us to calculate the concentration or purity of chemicals, analyze specific chemical characteristics, and follow and measure chemical reactions in real time.
Table of Contents
- Q. What is the purpose of measuring the absorbance of a solution at different wavelengths?
- Q. Why do we want to use a particular wavelength when determining the absorption of a particular chemical species is it important to measure absorbance for all wavelengths from 400 700 nm?
- Q. Why does a particular compound absorb light at a given wavelength?
- Q. Why do Solutions absorb light?
- Q. How does concentration affect how much light is absorbed and transmitted through the solution?
- Q. Why is beer-Lambert law used?
- Q. How is Beer’s law used in real life?
- Q. Why absorbance increases with concentration?
- Q. Why Beer Lambert law is not obeyed at high concentrations?
- Q. How accurate is Beer’s law?
- Q. Why monochromatic light is used in beer Lambert law?
- Q. What is the maximum absorbance?
- Q. How do you calculate absorbance?
- Q. How does changing wavelength affect absorbance?
- Q. Does higher wavelength mean more absorbance?
- Q. Does absorbance depend on wavelength?
- Q. Does pH affect Isosbestic point?
- Q. Does absorbance increase with temperature?
- Q. What does absorbance value tell you?
- Q. What does a high absorbance value mean?
- Q. What is negative absorbance?
Q. Why do we want to use a particular wavelength when determining the absorption of a particular chemical species is it important to measure absorbance for all wavelengths from 400 700 nm?
When selecting a wavelength for measurement, keep in mind that a wavelength at maximum absorbance provides maximum sensitivity, but the smallest concentration range, while a wavelength with a smaller absorbance would provide less sensitivity, but a larger concentration range to be measured in the experiment.
Q. Why does a particular compound absorb light at a given wavelength?
Each wavelength of light has a particular energy associated with it. If that particular amount of energy is just right for making one of these energy jumps, then that wavelength will be absorbed – its energy will have been used in promoting an electron.
Q. Why do Solutions absorb light?
The soda is a solution; it has lots of molecules (the solute) dissolved in a solvent (the water). Light is composed of photons. As photons shine through the solution, some of the molecules catch the photons. They absorb the light.
Q. How does concentration affect how much light is absorbed and transmitted through the solution?
The absorbance is directly proportional to the concentration of the solution. Some light is absorbed as it passes through a solution so less light is transmitted. Doubling the concentration doubles the absorbance. Doubling the path length doubles the absorbance.
Q. Why is beer-Lambert law used?
The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution, which enables the concentration of a solution to be calculated by measuring its absorbance.
Q. How is Beer’s law used in real life?
By comparing the spectra of suspected toxins with those from the crime scene, the nature of the poison can be determined. Once the identity of the poison is determined, Beer’s law can be used to determine the concentration of poison in the tainted wine.
Q. Why absorbance increases with concentration?
If the concentration of solution is increased, then there are more molecules for the light to hit when it passes through. As the concentration increases, there are more molecules in the solution, and more light is blocked. Therefore, the absorbance is directly proportional to the concentration.
Q. Why Beer Lambert law is not obeyed at high concentrations?
The Beer-Lambert Law will not be obeyed if the photons of light striking the detector do not all have an equal chance of absorption by the sample. This can happen if they have different absorption coefficients, different path lengths through the sample, or if they encounter different concentrations of sample molecules.
Q. How accurate is Beer’s law?
Beer’s Law is a simple linear proportionality between concentration and absorbance. Inexpensive spectrophotometers may only be accurate up to absorbances of 1, but higher quality ones may be capable of accurately measuring absorbances of 3.
Q. Why monochromatic light is used in beer Lambert law?
Strict adherence to Beer’s law is observed only with truly monochromatic radiation. Monochromators are used to isolate portions of the output from continuum light sources, hence a truly monochromatic radiation never exists and can only be approximated, i.e. by using a very narrow exit slit on the monochromator.
Q. What is the maximum absorbance?
For most spectrometers and colorimeters, the useful absorbance range is from 0.1 to 1. Absorbance values greater than or equal to 1.0 are too high. If you are getting absorbance values of 1.0 or above, your solution is too concentrated.
Q. How do you calculate absorbance?
Absorbance (A) is the flip-side of transmittance and states how much of the light the sample absorbed. It is also referred to as “optical density.” Absorbance is calculated as a logarithmic function of T: A = log10 (1/T) = log10 (Io/I).
Q. How does changing wavelength affect absorbance?
When you are taking an absorbance spectrum, and measuring the absorbance at different wavelengths, this is the only factor that is changing, as the concentration of the solution remains the same, and so does the pathlength.
Q. Does higher wavelength mean more absorbance?
One important consideration is the wavelength of radiation to use for the measurement. Remember that the higher the molar absorptivity, the higher the absorbance. What this also means is that the higher the molar absorptivity, the lower the concentration of species that still gives a measurable absorbance value.
Q. Does absorbance depend on wavelength?
This is Beer’sLaw: at constant path length, the absorbance is directly proportional to the concentration of absorbing material. in which b is the path length, C is the concentration, and a is a constant which depends on the wavelength of the light, the absorbing material, and the medium (solvent and other components).
Q. Does pH affect Isosbestic point?
The isosbestic point is the point on the graph of absorbance vs. pH where the molar absorption coefficients of the species in equilibrium are the same.
Q. Does absorbance increase with temperature?
The absorption and fluorescence spectra of peroxidase solutions is independent of temperature in the range from 10 to 45 degrees C. Above 45 degrees C the absorption decreases in the visible range and increases in the ultraviolet. The intensity of fluorescence decreases with the increase of temperature.
Q. What does absorbance value tell you?
D. Absorbance is a measure of the quantity of light absorbed by a sample. It is also known as optical density, extinction, or decadic absorbance. If all light passes through a sample, none was absorbed, so the absorbance would be zero and the transmission would be 100%.
Q. What does a high absorbance value mean?
When you get very high absorbance (>1.5), it means that most of the light are absorbed by the sample and only small amount of the light detected by detector.
Q. What is negative absorbance?
A negative absorbance means that the the intensity of light passing through the sample is greater than the intensity of light passing through the reference. If the experiment is performed correctly, a negative absorbance may have an important significance.