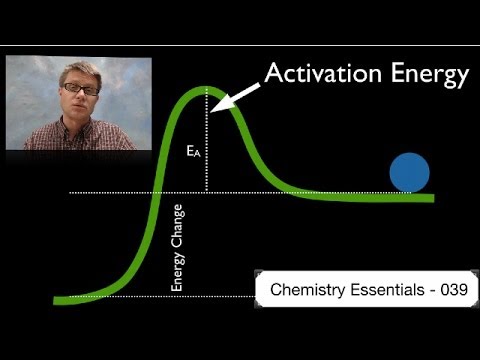
Negative activation energy for a given chemical reaction means that the rate of this reaction decreases with increasing temperature.
Q. What energy is activation?
Activation energy, in chemistry, the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo chemical transformation or physical transport. …
Table of Contents
- Q. What energy is activation?
- Q. Can activation energy becomes zero?
- Q. What is the difference between activation energy and free energy?
- Q. What is required for a reaction to occur?
- Q. What three conditions are required for a reaction to occur?
- Q. How does a reaction happen?
- Q. Is Gibbs free energy is negative?
Q. Can activation energy becomes zero?
It means that the rate of reaction, when activation energy is zero will have the value equal to the value of the collision frequency not temperature. The activation energy of a reaction is zero. The rate constant of the reaction is nearly independent of temperature. The correct option is (D).
Q. What is the difference between activation energy and free energy?
Activation energy Energy must be added to the reactants to overcome the energy barrier, which is recovered when products are formed. The activation energy is distinct from the ΔG, or free energy difference between the reactants and products.
Q. What is required for a reaction to occur?
According to the collision theory, the following criteria must be met in order for a chemical reaction to occur: Molecules must collide with sufficient energy, known as the activation energy, so that chemical bonds can break. Molecules must collide with the proper orientation.
Q. What three conditions are required for a reaction to occur?
Three things must happen for a reaction to occur.
- Molecules must collide.
- Molecules must collide with enough energy to begin to break the old bonds so new bonds can form. ( Remember activation energy)
- Molecules must collide with the correct orientation.
Q. How does a reaction happen?
Reactions occur when two or more molecules interact and the molecules change. Bonds between atoms are broken and created to form new molecules. That’s it. When you are trying to understand chemical reactions, imagine that you are working with the atoms.
Q. Is Gibbs free energy is negative?
Gibbs free energy is negative for a spontaneous reaction (only). It can be positive as well, for reactions that are not spontaneous.