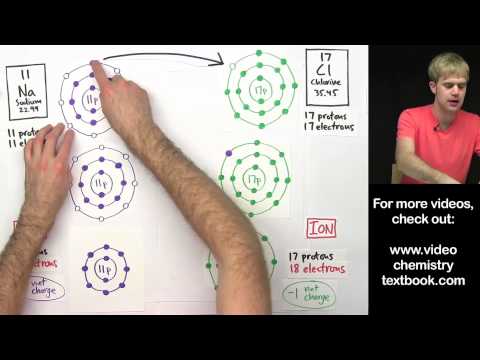
Q. What happens to valence electrons in ionic bond?
Ionic bonding is the complete transfer of valence electron(s) between atoms. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
Q. How do you find the number of valence electrons in a compound?
The number of valence electrons for molecules can be calculated by adding the valence electrons of all the atoms that form that respective molecule. 2⋅1+1⋅6=8 valence electroncs.
Table of Contents
- Q. What happens to valence electrons in ionic bond?
- Q. How do you find the number of valence electrons in a compound?
- Q. How do ionic compounds stay together?
- Q. Which compound has 24 valence electrons?
- Q. What is the valence of Na?
- Q. How can we find Valency of oxygen?
- Q. What is the Valency of oxygen negative or positive?
- Q. How do you find Valency of oxygen?
- Q. What is the Valency of H and O in h2o?
- Q. How many valence electrons will an atom of oxygen have?
- Q. What is Ion and give examples?
Q. How do ionic compounds stay together?
Ionic Bonding. An ionic bond is held together by the electrostatic attraction between ions that are near one another. Electrostatic attraction is the attraction between atoms that have opposite charge and holds the atoms together in ionic bonds.
Q. Which compound has 24 valence electrons?
There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
Q. What is the valence of Na?
11 1
Q. How can we find Valency of oxygen?
Atomic Number of oxygen is 8. So, electronic Configuration of oxygen= 2, 6. So.. valency is 8-6 = 2.
Q. What is the Valency of oxygen negative or positive?
The valency of oxygen is -2. This means oxygen needs to gain or share two electrons for stability. Remember, electrons are negative, which is why the…
Q. How do you find Valency of oxygen?
Oxygen has 8 valence electrons. Hence it belongs to group 6 of the periodic table. This means it has a total of 2 electrons in the first shell (or K shell), and 6 electrons in the first shell (or K shell). Hence it has a valency of 6.
Q. What is the Valency of H and O in h2o?
Hence, Valency of H in H2 is +1. In H20 molecule, O is more electronegative than Hydrogen and Hydrogen has one valence electron Oxygen gain two electrons from each Hydrogen atom. Hence, Valency of H in H20 is +2.
Q. How many valence electrons will an atom of oxygen have?
six valence electrons
Q. What is Ion and give examples?
An ion is a positively or negatively charged atom or groups of atoms. There are two types of ions: For example, Sodium (Na) atomic number is 11. So, there is one electron in the valence shell. To stabilize the atom, sodium loses one electron and is a deficit of the negative charge.