Q. What happens when ethyl alcohol is heated?
When ethanol is heated with an excess of concentrated sulphuric acid at a temperature of 170°C, it produces ethene as final product. The concentrated acid acts only as a dehydrating agent and it is recovered unchanged.
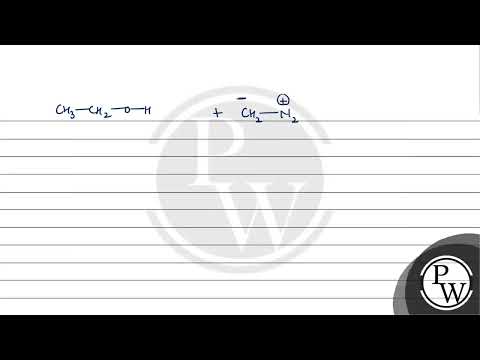
Q. What happens when ethyl alcohol?
When ethyl alcohol reacts with acetic acid, the hydroxyl group of the alcohol and the hydrogen of the acid react together and get eliminated as a water molecule. The ethyl (C2H5) groups of ethyl alcohol and acetic acid react together to form an ester. The ester, thus, formed is called ethyl ethanoate or ethyl acetate.
Table of Contents
- Q. What happens when ethyl alcohol is heated?
- Q. What happens when ethyl alcohol?
- Q. What happens when ethyl alcohol reacts with H2SO4?
- Q. When an ethyl alcohol is heated to 140 C with conc H2SO4 the product formed is?
- Q. What happens when ethyl alcohol is heated with excess of conc h2so4?
- Q. What happens when ethyl alcohol heated with concentrated h2so4 at 413k?
- Q. What happens when ethyl alcohol reacts with pcl5?
- Q. When ethyl alcohol is distilled with conc h2so4 under reduced pressure the product is?
- Q. What kind of alcohol is denatured alcohol?
- Q. What can I use in place of denatured alcohol?
- Q. Which is more effective ethyl or isopropyl alcohol?
- Q. What is the difference between ethyl rubbing alcohol and isopropyl alcohol?
- Q. What is the difference of isopropyl alcohol and ethyl alcohol?
- Q. Can I use ethyl alcohol to clean?
- Q. What happens when you mix ethyl and isopropyl alcohol?
Q. What happens when ethyl alcohol reacts with H2SO4?
When Ethyl alcohal , that us commonly known as Ethanol reacts with Conc. H2SO4 , Concetrated Sulphuric acid , then it forms Ethene along with water . Here H2SO4 is a catalyst , whic works like a dehydrating agent .
Q. When an ethyl alcohol is heated to 140 C with conc H2SO4 the product formed is?
How does this reaction happen? At 140°C in the presence of sulfuric acid two molecules of ethanol are dehydrated (lose a molecule of water) to form ether. The sulphuric acid is regenerated and reused. Above about 160°C (320°F) ethene is produced instead, by loss of water from just one molecule of ethanol.
Q. What happens when ethyl alcohol is heated with excess of conc h2so4?
Q. What happens when ethyl alcohol heated with concentrated h2so4 at 413k?
When ehanol is heated with concentrated sulphuric acid at 443 K, dehydration takes place and ethene is formed. In this reaction concentrated sulphuric acid acts as a dehydrating agent.
Q. What happens when ethyl alcohol reacts with pcl5?
When ethanol reacts with phosphorus pentachloride in the presence of heat ethyl chloride hydrochloric acid and phosphoryl chloride is produced.
Q. When ethyl alcohol is distilled with conc h2so4 under reduced pressure the product is?
Ethanol reacts with sulphuric acid at 383K to form ethyl hydrogen sulphate which under reduced pressures transforms into diethyl sulphate. At 413K, the product formed in this reaction is diethyl ether.
Q. What kind of alcohol is denatured alcohol?
Denatured alcohol is ethanol (ethyl alcohol) made unfit for human consumption by adding one or more chemicals (denaturants) to it. Denaturing refers to removing a property from the alcohol (being able to drink it), not to chemically altering or decomposing it, so denatured alcohol contains ordinary ethyl alcohol.
Q. What can I use in place of denatured alcohol?
If you have trouble finding denatured alcohol, there are plenty of alternatives that you may use. What can you use in place of denatured alcohol? Some of the options include; methanol, acetone, grain alcohol, and absolute isopropyl alcohol.
Q. Which is more effective ethyl or isopropyl alcohol?
Does alcohol kills germs and viruses? Isopropyl alcohol is effective against viruses such as FCV at 40% – 60% concentrations. Ethanol however, is more effective at 70% – 90% concentrations against FCV.
Q. What is the difference between ethyl rubbing alcohol and isopropyl alcohol?
Answer: Ethyl alcohol (ethanol), or “grain” alcohol, is used widely all over the world in beverages as an intoxicating agent. It is the most popular legalized drug in the world. Isopropyl alcohol, or “rubbing” alcohol, is sold in pharmacies as an antiseptic and should never be consumed.
Q. What is the difference of isopropyl alcohol and ethyl alcohol?
Ethanol is colourless and flammable liquid that is soluble in water. IPA or Isopropyl alcohol is also a colourless and flammable liquid but has a slightly difference chemical composition with a formula of C 3H 8O.
Q. Can I use ethyl alcohol to clean?
Ethyl alcohol is a great choice for cleaning electronics in the laboratory because it’s inexpensive, mineral-free, and evaporates quickly. While ethanol isn’t a strong solvent, it is typically sufficiently powerful to clean sensitive electronics which may have accumulated light layers of corrosion or dirt.
Q. What happens when you mix ethyl and isopropyl alcohol?
Rubbing alcohol contains ethanol and isopropyl, which when mixed with bleach creates chloroform, a toxic compound that emits toxic and corrosive fumes.