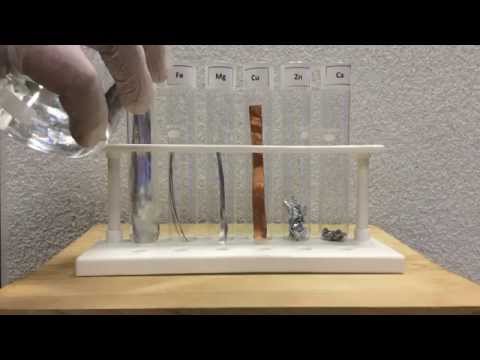
Iron sulfide reacts with hydrochloric acid, releasing the malodorous (rotten egg smell) and very toxic gas,hydrogen sulphide. When powdered ferrous sulphide (FeS) is dropped in dilute hydrochloric acid(HCl) , they react, bubbles of gas are seen in the acid.
Q. What is the product of FeS HCl?
Iron sulfide reacts with hydrochloric acid, releasing hydrogen sulfide: FeS + 2 HCl → FeCl2 + H2S.
Table of Contents
- Q. What is the product of FeS HCl?
- Q. What type of reaction is Fe O2 → Fe2O3?
- Q. What happens when dilute Sulphuric acid is added to FeS?
- Q. What will happen if HCl is added to FeS?
- Q. What do you observe when dilute hydrochloric acid is added to iron sulphide?
- Q. What do you observe when hydrochloric acid is added to agno3 solution?
- Q. When dilute hydrochloric acid is added to iron II sulphide the gas evolved will?
- Q. How do you know if gas is HCl?
- Q. Which gas is evolved when dilute hydrochloric acid reacts with iron 2 sulphide?
- Q. What happens when dilute hydrochloric acid is added to zinc sulphide?
- Q. What happens when dilute hydrochloric acid is added to zinc metal give balanced equation?
- Q. What happens when dilute hydrochloric acid is added to sodium thiosulphate?
- Q. What do you observe when dilute hydrochloric acid is added to zinc?
- Q. Does magnesium react with dilute hydrochloric acid?
- Q. What will be your observation when dilute hydrochloric acid is added to a barium chloride?
- Q. What happens when dilute solution of hydrochloric acid and caustic soda are mixed together prepare an experiment?
- Q. What happens when lead nitrate solution is mixed with dilute hydrochloric acid and heated?
- Q. What do you observe when lead nitrate solution is mixed with dilute Sulphuric acid?
- Q. What happens when lead nitrate crystals are heated?
- Q. What happens when Pb NO3 2 reacts with HCl?
- Q. Does PB dissolve in HCl?
- Q. What is the product of Pb NO3 2 HCl?
Q. What type of reaction is Fe O2 → Fe2O3?
Fe + O2 → Fe2O3 is the unbalanced form of chemical reaction. Fe + 3O2→ Fe2O3 there are now 6 O atoms on the left, there must also be 6 O atoms on the right. Balance the rest of the equation.
Q. What happens when dilute Sulphuric acid is added to FeS?
a. When dilute sulphuric acid is added to a mixture of iron fillings and sulphur powder, reaction occusrs between dilute sulphuric acid and iron filings that forms ferrous sulphate and evolution of hydrogen. FeS formed reacts with sulphuric acid to form ferrous sulphate and release hydrogendisulphide gas.
Q. What will happen if HCl is added to FeS?
When dilute HCI is added to part A, FeS will react with dil HCI to form H2S gas which has smell of rotten eggs and will turn lead acetate paper black. When dil, HCI is added to it, iron filings react with dill.
Q. What do you observe when dilute hydrochloric acid is added to iron sulphide?
When dilute hydrochloric acid is added to the compound of Iron sulphide, it releases hydrogen sulphide gas. It smells bad like the smell of rotten eggs.
Q. What do you observe when hydrochloric acid is added to agno3 solution?
Hydrochloric acid is added to silver nitrate solution. When a hydrochloric acid is added to silver nitrate solution then a curdy white ppt is formed.
Q. When dilute hydrochloric acid is added to iron II sulphide the gas evolved will?
Explanation: Iron sulfide FeS reacts with Hydrochloric acid HCl to yield H2S, Hydrogen Sulphide, which turns acetate paper black or filter paper moistened with lead (ii) trioxonitrate black.
Q. How do you know if gas is HCl?
Hydrochloric acid is found as a white precipitate forms. Hydrogen chloride is a colourless or mildly yellow, corrosive, nonflammable gas at room temperature that is thicker than air and has a powerful unpleasant odour. Hydrogen chloride forms thick white corrosive vapours upon contact to oxygen.
Q. Which gas is evolved when dilute hydrochloric acid reacts with iron 2 sulphide?
hydrogen sulphide
Q. What happens when dilute hydrochloric acid is added to zinc sulphide?
Zinc reacts with dilute HCl to give a metallic zinc chloride with the evolution of hydrogen gas.
Q. What happens when dilute hydrochloric acid is added to zinc metal give balanced equation?
The reaction between zinc and HCl is given by the symbolic equation Zn + HCl → ZnCl2 + H2. Note that H2 is hydrogen gas.
Q. What happens when dilute hydrochloric acid is added to sodium thiosulphate?
When sodium thiosulphate is added to a solution of hydrochloric acid, an insoluble precipitate of sulphur (S) is formed. This happens because of the precipitates of elemental sulphur that are being formed, which are insoluble and eventually cloud the water.
Q. What do you observe when dilute hydrochloric acid is added to zinc?
Answer: (d) A colourless and odourless gas is evolved. A colourless and odourless gas is produced with bubbles when diluting hydrochloric acid is introduced to the granulated zinc placed in the test tube.
Q. Does magnesium react with dilute hydrochloric acid?
Magnesium reacts with dilute hydrochloric acid in a conical flask which is connected to an inverted measuring cylinder in a trough of water. The volume of hydrogen gas produced is measured over a few minutes, and the results are used to plot a graph.
Q. What will be your observation when dilute hydrochloric acid is added to a barium chloride?
Dilute Hydrochloric acid is added to Lead nitrate Solution and the mixture is heated. When dil. HCl is added to lead nitrate solution and heated, it forms a white precipitate of lead chloride. A white precipitate of barium sulphate forms when barium chloride is mixed with sodium sulphate.
Q. What happens when dilute solution of hydrochloric acid and caustic soda are mixed together prepare an experiment?
Answer. Answer: Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (the salt) and water. HCl+NaOH→H2O+NaCl.
Q. What happens when lead nitrate solution is mixed with dilute hydrochloric acid and heated?
When dilute HCl is added to lead nitrate solution and heated, it forms a white precipitate of lead chloride and nitric acid. In this double displacement, a reaction takes place. On heating, the precipitate dissolves slowly to give a clear solution because lead chloride is fairly soluble in hot water.
Q. What do you observe when lead nitrate solution is mixed with dilute Sulphuric acid?
When dilute hydrochloric acid is added to lead nitrate solution. white ppt of lead chloride appears.
Q. What happens when lead nitrate crystals are heated?
Lead nitrate crystals on strong heating decompose to form lead monoxide, nitrogen dioxide gas and oxygen gas.
Q. What happens when Pb NO3 2 reacts with HCl?
In this reaction when dilute HCl is added to lead nitrate solution, lead chloride and nitric acid will form. The substances that undergo chemical change in the above reaction, lead nitrate, and dilute hydrochloric acid are the reactants. The new substance lead chloride and nitric acid are products.
Q. Does PB dissolve in HCl?
Lead(II) chloride
| Names | |
|---|---|
| Boiling point | 950 °C (1,740 °F; 1,220 K) |
| Solubility in water | 10.8 g/L (20 °C) |
| Solubility product (Ksp) | 1.7×10−5 (20 °C) |
| Solubility | slightly soluble in dilute HCl, ammonia; insoluble in alcohol Soluble in hot water as well as in presence of alkali hydroxide Soluble in concerntrated HCl (>6M) |
Q. What is the product of Pb NO3 2 HCl?
Pb(No3)2 + HCl = PbCl2 + HNo3 – Chemical Equation Balancer.