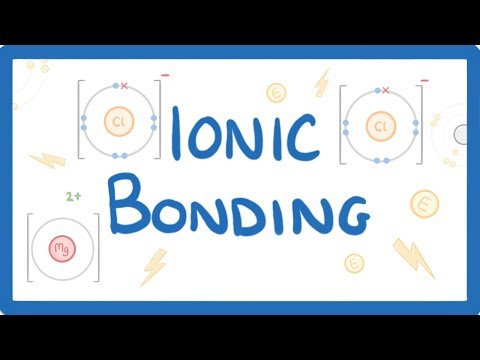
When an electron of sodium atom is transferred to chlorine atom it forms sodium chloride. This chemical bonding gives Na+ and Cl-. The electrons are of opposite charges so they are attracted to each other and the end result is the formation of NaCl.
Q. What happens when you mix sodium and chlorine?
If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. This reaction is highly favorable because of the electrostatic attraction between the particles. In the process, a great amount of light and heat is released.
Table of Contents
- Q. What happens when you mix sodium and chlorine?
- Q. Why do sodium and chlorine form an ionic bond?
- Q. Which two elements will most likely form an ionic bond?
- Q. What is most likely to form a ion?
- Q. Can magnesium and bromine form ionic bonds?
- Q. Which element is most likely to form an ionic bond with potassium?
- Q. Does potassium bond with sodium?
- Q. Does sodium and oxygen form an ionic bond?
- Q. Which one of the following is most likely to contain an ionic bond?
- Q. Which of the following does not contain ionic bonds?
- Q. How do you know if something is more ionic?
- Q. How can you tell if its ionic or covalent?
Q. Why do sodium and chlorine form an ionic bond?
Sodium has 1 electron in its outermost shell, and chlorine has 7 electrons. If sodium can transfer it’s “spare” electron to chlorine (as shown above), both atoms will satisfy their full outer shell requirements, and an ionic bond will be formed.
Q. Which two elements will most likely form an ionic bond?
Which two elements will most likely form an ionic bond? Iodine and potassium form an ionic bond. Iodine has seven electrons in its outer shell, and potassium has one electron in its outer shell.
Q. What is most likely to form a ion?
The elements that are most likely to form 2− ions are the group 16 elements. Their atoms have 6 valence electrons, and need 2 more to have a full valence shell of 8 electrons in order to become stable. When they gain 2 electrons in order to have 8 valence electrons, an octet, they gain a 2− charge.
Q. Can magnesium and bromine form ionic bonds?
For example, magnesium and bromine form an ionic bond. Magnesium has an oxidation number of +2 and bromine has an oxidation number of -1.
Q. Which element is most likely to form an ionic bond with potassium?
Answer : The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and chlorine.
Q. Does potassium bond with sodium?
Explain why sodium and potassium will or will not react to form a bond with each other. Sodium and Potassium belong to the same group in the periodic tabel (Group I). So, a chemical reaction between both these elements is not going to establish octant state for either of them. SO, they dont react with each other.
Q. Does sodium and oxygen form an ionic bond?
Sodium will react with oxygen and form an ionic compound.
Q. Which one of the following is most likely to contain an ionic bond?
Explanation: The compound most likely to contain an ionic bond is CaCl₂ because it is the only specie with a metal and non-metal. The metal here is Ca and non-metal is Cl. In this bond type, there is a transfer of electrons from the metal to the non-metal.
Q. Which of the following does not contain ionic bonds?
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. HCl, H-atom and Cl-atom shares one electron pair to form covalent bond.
Q. How do you know if something is more ionic?
If the electronegativity difference between the two elements is greater than or equal to 1.7, the bond is more ionic than covalent. There are several formulas for calculating percent ionic character. They give a fair correlation with ionic character as determined by dipole moments.
Q. How can you tell if its ionic or covalent?
By definition, an ionic bond is between a metal and a nonmetal, and a covalent bond is between 2 nonmetals. So you usually just look at the periodic table and determine whether your compound is made of a metal/nonmetal or is just 2 nonmetals.