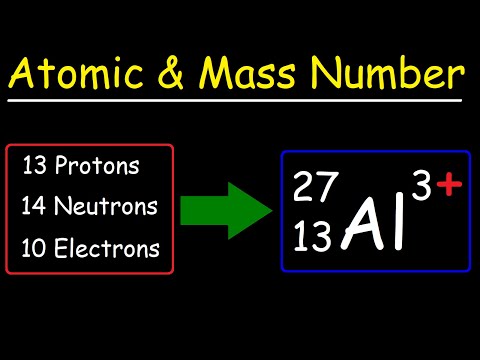
Q. What information can you use to determine the atomic number?
Atoms of each element contain a characteristic number of protons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); the number of protons in an atom is called the atomic number. In contrast, the number of neutrons for a given element can vary.
Q. What information do you need to calculate atomic mass for an element?
For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom.
Table of Contents
- Q. What information can you use to determine the atomic number?
- Q. What information do you need to calculate atomic mass for an element?
- Q. What information can you get from an element’s mass number?
- Q. What two things can you work out from the atomic number of an element?
- Q. Which element has a symbol that starts with a letter different from the first one?
- Q. What element has the atomic mass of 20?
- Q. Will silicone cause metal to rust?
- Q. Will silicone stick to galvanized metal?
- Q. Do you need to seal galvanized metal?
- Q. Does silicone hold metal together?
- Q. What is the best metal roof sealant?
- Q. Do I need to seal metal roof?
- Q. Does roof coating stop leaks?
- Q. How do you seal an old metal roof?
- Q. Can you put tar on a metal roof?
- Q. Can you coat a metal roof?
- Q. Why do screws back out on metal roof?
- Q. How do you find the atomic mass of an unknown element?
- Q. How do we calculate relative atomic mass?
- Q. How do you figure out the atomic mass?
- Q. What means mass number?
- Q. Where is most of the atoms mass?
- Q. Do electrons have significant mass?
Q. What information can you get from an element’s mass number?
An element’s mass number (A) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number.
Q. What two things can you work out from the atomic number of an element?
Calculating numbers of subatomic particles number of protons = atomic number. number of electrons = atomic number. number of neutrons = mass number – atomic number.
Q. Which element has a symbol that starts with a letter different from the first one?
Periodic Table Scavenger Hunt Part 2
| Question | Answer |
|---|---|
| 6. What is the atomic mass of carbon? | bessemer |
| 7. What do you call the element series from atomic number 57-71? | bessemer |
| 8. Which element has a symbol that starts with a letter different from the first one in its name: aluminum, copper, gold, rhenium? | bessemer |
Q. What element has the atomic mass of 20?
Ca
Q. Will silicone cause metal to rust?
Silicone itself doesn’t cause rust, but it traps any moisture that gets under it. And rust absorbs moisture like a sponge and perpetuates itself. If you silicone over one side of rusted sheet metal, or a blind seam, you’ve made matters worse.
Q. Will silicone stick to galvanized metal?
Neutral-cure silicones are used successfully in metal fabrication projects. Acid-cure silicones, on the other hand, can have a corrosive reaction with galvanized metals. On the other hand, acid-cure silicone can etch the surface of aluminum and increase adhesion.
Q. Do you need to seal galvanized metal?
Over time, any type of metal is prone to rust or corrosion, it’s inevitable. It’s very important to protect the surface of your galvanized steel to extend its lifespan. Using a clear coat extends the lifespan of the steel and makes it look better.
Q. Does silicone hold metal together?
Silicone sealants can be used to bond many common materials, including plastic, metal, glass, and ceramic.
Q. What is the best metal roof sealant?
For a quality product that offers durability, choose Titebond® WeatherMaster™ Metal Roof Sealant. Titebond is not your standard sealant. It is specifically formulated to outperform the other sealants including urethanes, silicones, VOC solvent, and tri-polymers.
Q. Do I need to seal metal roof?
For a newly constructed metal roof, a metal roof sealer is required. Typically butyl seam tape is positioned over the joints and seams between the metal panels and butyl caps are placed over all nails and fasteners to ensure proper sealant.
Q. Does roof coating stop leaks?
Does roof coating stop leaks? Yes, a silicone flat roof coating will seal up and stop existing roof leaks and there is no need to locate and identify each leak. A roofing contractor that knows How to apply flat roof coating can cover the entire roof which will protect the entire roof while stopping leaks.
Q. How do you seal an old metal roof?
How to Seal a Tin Roof
- Power-wash your roof to remove as much dirt and debris as possible.
- Seal around nail holes and other openings in the roof with outdoor caulk, just as you would seal the joints.
- Cut in tight areas and corners of the roof with sealant using a paintbrush.
Q. Can you put tar on a metal roof?
Tar can actually be used on a metal roof, and many people slop the black goop onto valleys, roof-to-wall joints and plumbing vents to prevent or stop leaks, notes William Kibbel III, a home inspector and restoration consultant who serves as vice president of the Tri-County Inspection Co.
Q. Can you coat a metal roof?
Metal roofs can be re-coated for a variety of reasons. Initially, they have a factory finish coating that may be galvanized, or Galvalume finished steel panels or fluoropolymer-based paint such as Kynar 500. However, as metal panels reach the end of their designed life expectancy, their coating becomes compromised.
Q. Why do screws back out on metal roof?
Thermal expansion and contraction of the metal roofing, and possibly movement of the substrate, can cause through-fasteners to loosen or back out over time. Metal roofing with loose or missing fasteners will suffer reduced wind resistance and possible moisture intrusion.
Q. How do you find the atomic mass of an unknown element?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Q. How do we calculate relative atomic mass?
Find the relative mass of any atom by adding the number of protons to the number of neutrons. Hydrogen has a relative atomic mass of 1, and carbon-12 has a relative atomic mass of 12. Isotopes of the same element have different numbers of neutrons, so you need to calculate for one specific isotope.
Q. How do you figure out the atomic mass?
Q. What means mass number?
Mass number, in nuclear physics, the sum of the numbers of protons and neutrons present in the nucleus of an atom.
Q. Where is most of the atoms mass?
nucleus
Q. Do electrons have significant mass?
An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom. The electron was discovered in 1897 by the English physicist J.J.