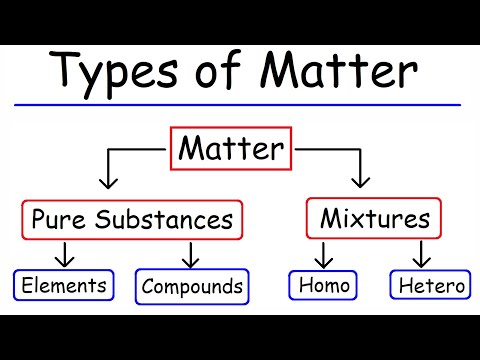
Elements: • A pure substance containing only one kind of atom . • An element cannot be separated into simpler materials (except during nuclear. reactions).
Q. What is a substance that contains only one type of atom?
Atoms are the building blocks of all matter. When a substance contains only one type of atom, it is called an element. An element is a pure substance and is made of only one type of atom; it cannot be broken down into a simpler substance.
Table of Contents
- Q. What is a substance that contains only one type of atom?
- Q. What is a single atom called?
- Q. Is a pure chemical substance consisting of only one type of atoms?
- Q. What are the two types of pure substances?
- Q. What are 2 types of mixtures?
- Q. What are two pure substances examples?
- Q. What is the two types of substances?
- Q. Which is a pure compound?
- Q. How can you say that one substance is different from other?
- Q. What is a pure element?
- Q. What is the most pure element?
- Q. What are the 10 examples of elements?
- Q. What is the best example of an element?
- Q. What elements are in everyday items?
- Q. What are examples of elements at home?
- Q. What objects are pure?
- Q. What are pure substances kids?
- Q. What are 5 examples of mixtures?
- Q. How do you know if a substance is pure?
- Q. What type of substance is ice?
- Q. Is milk a mixture or a pure?
Q. What is a single atom called?
Some elements are monatomic, meaning they are made of a single (mon-) atom (-atomic) in their molecular form. Helium (He, see Fig. 2.8) is an example of a monatomic element.
Q. Is a pure chemical substance consisting of only one type of atoms?
A chemical element is a pure substance that consists of one type of atom. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element.
Q. What are the two types of pure substances?
The two main types of pure substances are compounds and elements. They consist of a single type of particle or compound.
Q. What are 2 types of mixtures?
Types of Mixtures There are two main categories of mixtures: homogeneous mixtures and heterogeneous mixtures.
Q. What are two pure substances examples?
Examples of Pure Substances All elements are mostly pure substances. A few of them include gold, copper, oxygen, chlorine, diamond, etc. Compounds such as water, salt or crystals, baking soda amongst others are also grouped as pure substances.
Q. What is the two types of substances?
Types of Substances Pure Substance: The substances that are free from any kind of mixture and contain only one kind of particle are pure substances. Examples of pure substances include iron, aluminum, silver, and gold. Mixtures: Substances that have two or more different particles are mixtures.
Q. Which is a pure compound?
A pure chemical compound is a chemical substance that is composed of a particular set of molecules or ions that are chemically bonded. Two or more elements combined into one substance through a chemical reaction, such as water, form a chemical compound.
Q. How can you say that one substance is different from other?
Different substances have different properties; density is one property that can be used to tell two substances apart. Density is a property that does not depend on the shape or size of an object.
Q. What is a pure element?
Chemists and nuclear scientists have different definitions of a pure element. In chemistry, a pure element means a substance whose atoms all (or in practice almost all) have the same atomic number, or number of protons. Nuclear scientists, however, define a pure element as one that consists of only one stable isotope.
Q. What is the most pure element?
Hydrogen is the most abundant element in the universe.
Q. What are the 10 examples of elements?
Pure Element Examples
- Hydrogen (H) – nonmetal.
- Helium (He) – nonmetal.
- Oxygen (O) – nonmetal.
- Neon (Ne) – nonmetal.
- Nitrogen (N) – nonmetal.
- Carbon (C) – reactive nonmetal.
- Silicon (Si) – metalloid.
- Magnesium (Mg) – alkaline earth metal.
Q. What is the best example of an element?
Examples of elements include iron, oxygen, hydrogen, gold, and helium. An important number in an element is the atomic number. This is the number of protons in each atom.
Q. What elements are in everyday items?
| ELEMENT | USES | |
|---|---|---|
| 1) | Aluminum | A light metal used in making airplanes, buildings, pots & pans, etc. |
| 2) | Bromine | Used in photography, medicines, insecticides, etc. |
| 3) | Calcium | A soft, metallic chemical element found in limestone, marble, chalk, etc. |
| 4) | Carbon | Found in coal, oil gas, living things, & inks |
Q. What are examples of elements at home?
What are 5 pure elements that can be found in your home?
- Argon and tungsten are in incandescent light bulbs.
- Mercury is in some thermostats and in switches in space heaters that turn off when tipped over.
- Copper is used in electrical wiring and in some water pipes.
- Carbon is in pencils.
- Phosphorous is on the tips of matches and ignites from the friction of striking them.
Q. What objects are pure?
Examples of pure substances include tin, sulfur, diamond, water, pure sugar (sucrose), table salt (sodium chloride) and baking soda (sodium bicarbonate). Crystals, in general, are pure substances. Tin, sulfur, and diamond are examples of pure substances that are chemical elements.
Q. What are pure substances kids?
A pure substance is a type of matter which exists in its most basic or purest form and cannot be broken down further. Examples of pure substances include water, gases like carbon dioxide, oxygen and metals like platinum, gold and silver.
Q. What are 5 examples of mixtures?
Here are a few more examples:
- Smoke and fog (Smog)
- Dirt and water (Mud)
- Sand, water and gravel (Cement)
- Water and salt (Sea water)
- Potassium nitrate, sulfur, and carbon (Gunpowder)
- Oxygen and water (Sea foam)
- Petroleum, hydrocarbons, and fuel additives (Gasoline)
Q. How do you know if a substance is pure?
A pure substance has a definite and constant composition — like salt or sugar. A pure substance can be either an element or a compound, but the composition of a pure substance doesn’t vary.
Q. What type of substance is ice?
Crystal methamphetamine (‘ice’, ice drug) is a stimulant drug, which means it speeds up the messages travelling between the brain and the body.
Q. Is milk a mixture or a pure?
Main compounds of milk are lactose and casein. And it is also called a colloidal mixture (i.e. in which one substance of microscopically dispersed insoluble or soluble particles is suspended throughout another substance). Therefore milk is regarded as a mixture not as a pure substance.