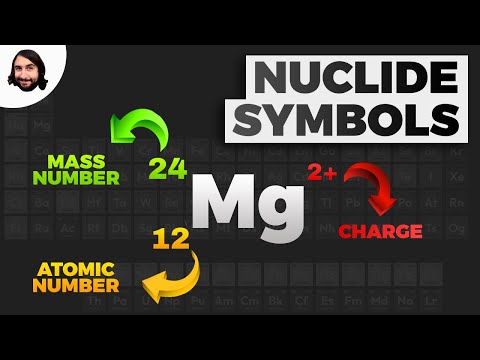
Q. What is an isotopic symbol?
These differing atoms are called isotopes. To write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a superscript to the left of the atomic symbol. The symbols for the two naturally occurring isotopes of chlorine are written as follows: 3517Cl and 3717Cl.
Q. How do you write carbon isotopes?
Standard nuclear notation shows the chemical symbol, the mass number and the atomic number of the isotope. Example: the isotopes of carbon. The element is determined by the atomic number 6. Carbon-12 is the common isotope, with carbon-13 as another stable isotope which makes up about 1%.
Table of Contents
Q. Will all the carbon-14 eventually disappear?
The half-life of carbon-14 is the amount of time it takes for one-half of the original amount to disappear by radioactive decay. After 50,000 years, a fossil won’t have any radiocarbon left in it. Carbon-14 will have all disappeared by radioactive decay.
Q. How do we know that carbon 14 dating is accurate?
By testing the amount of carbon stored in an object, and comparing to the original amount of carbon believed to have been stored at the time of death, scientists can estimate its age.
Q. How much of bone is carbon?
Considering that collagen contains about 40–45% carbon, 250 mg of bone are necessary to provide enough carbon for a regular-sized graphite target of 1 mg.
Q. Do bones have carbon in them?
Any carbon-containing material that may affect the carbon 14 content of bones is considered a contaminant. Considering that bones are often found surrounded by different kinds of organic matter, bones are arguably one of the most highly contaminated samples submitted to AMS labs for radiocarbon dating.
Q. Which mineral is present in bone?
Calcium
Q. Is there carbon 14 in fossils?
Carbon-14 is a radioactive isotope of carbon. The short half-life of carbon-14 means its cannot be used to date extremely old fossils. Levels of carbon-14 become difficult to measure and compare after about 50,000 years (between 8 and 9 half lives; where 1% of the original carbon-14 remains undecayed).