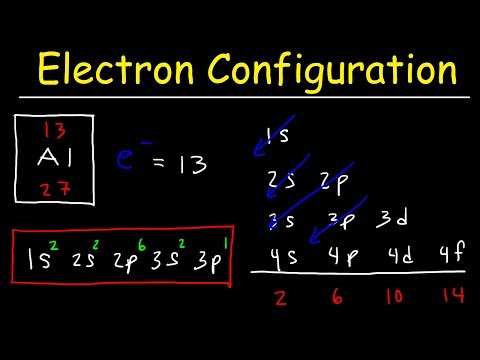
Q. What is coefficient in electron configuration?
Essentially, the coefficients that are used in electron configurations are meant to represent the energy level of the orbitals containing the specific number of electrons within a unique periodic element. In this case, The number 4 would indicate the electrons’ energy level (n=4) location.
Q. What is the coefficient in electron configuration of argon?
Therefore the Argon electron configuration will be 1s22s22p63s23p6.
Table of Contents
- Q. What is coefficient in electron configuration?
- Q. What is the coefficient in electron configuration of argon?
- Q. What do superscripts in an electron configuration represent?
- Q. How do you calculate electron configuration?
- Q. What is the difference between the ground and excited state of electron positions?
- Q. What is the first excited state?
- Q. What happens when electrons move from the excited to ground state?
- Q. What happens after an electron absorbs light?
- Q. How do photons excite electrons?
- Q. Why do electrons in the first shell have less potential energy?
- Q. What is the energy of an electron in the first shell?
- Q. Which electron shell has highest power?
Q. What do superscripts in an electron configuration represent?
Electron configuration notation simplifies the indication of where electrons are located in a specific atom. Superscripts are used to indicate the number of electrons in a given sublevel.
Q. How do you calculate electron configuration?
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
Q. What is the difference between the ground and excited state of electron positions?
The main difference between ground state and excited state is that ground state is a state whereas electrons in a system are in the lowest possible energy levels whereas excited state is any state of the system that has a higher energy than the ground state.
Q. What is the first excited state?
Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on. Remember that there is a maximum energy that each electron can have and still be part of its atom.
Q. What happens when electrons move from the excited to ground state?
When an atom is in an excited state, the electron can drop all the way to the ground state in one go, or stop on the way in an intermediate level. Electrons do not stay in excited states for very long – they soon return to their ground states, emitting a photon with the same energy as the one that was absorbed.
Q. What happens after an electron absorbs light?
When an electron is hit by a photon of light, it absorbs the quanta of energy the photon was carrying and moves to a higher energy state. Electrons therefore have to jump around within the atom as they either gain or lose energy.
Q. How do photons excite electrons?
Photons are electromagnetic waves that propagate in wave packet. Those wave packet carry a defined quantized amount of energy. When a photon interact with an electron it will give away its energy to the electron. The electron will have more energy and hence a larger velocity.
Q. Why do electrons in the first shell have less potential energy?
Explain why electrons in the first electron shell have less potential energy than electrons in higher electron shells. Because they are closer to the pulling of the nucleus. Explain how electrons can move from one energy shell to another. Describe what is meant by the ground state and the excited state of an electron.
Q. What is the energy of an electron in the first shell?
The energy of an electron in its nth orbit = -13.6/n2. PE in the first excited state = -6.8 eV.
Q. Which electron shell has highest power?
outermost shell