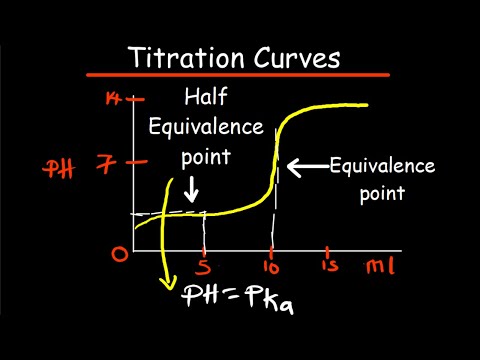
Equivalence point: point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution.
Q. Is the equivalence point always 7?
At the equivalence point, all of the weak acid is neutralized and converted to its conjugate base (the number of moles of H+ = added number of moles of OH–). However, the pH at the equivalence point does not equal 7.
Table of Contents
- Q. Is the equivalence point always 7?
- Q. How do you find the equivalence point on a titration curve?
- Q. How do you find the concentration at the equivalence point?
- Q. Does equivalence point equal concentration?
- Q. How do you find the equivalence point on a graph?
- Q. How do you find the half equivalence point on a graph?
- Q. What is the half equivalence point?
- Q. How do you find the pH at the half equivalence point?
- Q. What is the equivalence point of HCl and NaOH titration?
- Q. What is the pH at the first equivalence point?
- Q. How do you calculate pKb?
- Q. What is the full form of pKb?
- Q. Is pKa equal to pH?
- Q. What is the difference between PKB and KB?
- Q. Does higher KB mean high pH?
- Q. What is KB expression?
- Q. What is a KB reaction?
- Q. How do you find the KB value?
- Q. What is the Ka of HCl?
- Q. How do you calculate ka?
- Q. What is the kb value for H2PO4?
- Q. Is KW a constant?
- Q. What is KW equilibrium constant?
- Q. What is the value of KW at 298 K?
- Q. Why does KW not include water?
Q. How do you find the equivalence point on a titration curve?
On the curve, the equivalence point is located where the graph is most steep. There is a fast and abrupt change of pH around this point, which can be observed by the color change the takes place during titration. At the equivalence point, an ICE table is required to determine volume and acidity.
Q. How do you find the concentration at the equivalence point?
Divide the number of moles of analyte present by the original volume of the analyte. For example, if the original volume of the analyte was 500 mL, divide by 1000 mL per L to obtain 0.5 L. Divide 0.01 moles of analyte by 0.5 L to obtain 0.02 moles per liter. This is the concentration or molarity.
Q. Does equivalence point equal concentration?
Key Takeaways: Equivalence Point In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. In a titration, the equivalence point is not the same as the endpoint.
Q. How do you find the equivalence point on a graph?
To find the equivalence point volume, we seek the point on the volume axis that corresponds to the maximum slope in the curve; that is, the first derivative should exhibit a maximum in the first derivative. Now move your cursor to point directly at one of your data points on the first derivative plot.
Q. How do you find the half equivalence point on a graph?
The inflection point, which is the point at which the lower curve changes into the upper one, is the equivalence point. After having determined the equivalence point, it’s easy to find the half-equivalence point, because it’s exactly halfway between the equivalence point and the origin on the x-axis.
Q. What is the half equivalence point?
Half Equivalence Point. The half equivalence point represents the point at which exactly half of the acid in the buffer solution has reacted with the titrant. The half equivalence point is relatively easy to determine because at the half equivalence point, the pKa of the acid is equal to the pH of the solution.
Q. How do you find the pH at the half equivalence point?
At the half-equivalence point, pH = pKa when titrating a weak acid. After the equivalence point, the stoichiometric reaction has neutralized all the sample, and the pH depends on how much excess titrant has been added. After equivalence point, any excess strong base KOH determines the pH.
Q. What is the equivalence point of HCl and NaOH titration?
The point at which exactly enough titrant (NaOH) has been added to react with all of the analyte (HCl) is called the equivalence point. Up to the equivalence point, the solution will be acidic because excess HCl remains in the flask.
Q. What is the pH at the first equivalence point?
That is, if pKa1 2 and pKa2 6, the pH of the solution at the first equivalence point should be 4.
Q. How do you calculate pKb?
To get the pKb of the base (B) you MUST subtract the pKa from 14. The reason for this is that the pOH is actually what equals the pKb. pKb = 14 – pKa H+ in EXCESS that has been added. No equilibrium calculations necessary.
Q. What is the full form of pKb?
| PKB | Protein Kinase B Medical » Physiology | Rate it: |
|---|---|---|
| PKB | Peruvian KnuckleBuster Sports | Rate it: |
| PKB | Produkt krajowy brutto Miscellaneous » Unclassified | Rate it: |
| PKB | Pot Kettle Black Internet » Chat | Rate it: |
| PKB | Personal Knowledge Base Computing » Software | Rate it: |
Q. Is pKa equal to pH?
The pKa is the pH value at which a chemical species will accept or donate a proton. The lower the pKa, the stronger the acid and the greater the ability to donate a proton in aqueous solution. The Henderson-Hasselbalch equation relates pKa and pH.
Q. What is the difference between PKB and KB?
The base dissociation constants are interpreted just like the acid dissociation constants. A large Kb value means a base has largely dissociated and indicates a strong base. A small pKb value indicates a strong base, while a large pKb value indicates a weak base.
Q. Does higher KB mean high pH?
A large Kb value indicates the high level of dissociation of a strong base. A lower pKb value indicates a stronger base.
Q. What is KB expression?
Ka and Kb values measure how well an acid or base dissociates. Higher values of Ka or Kb mean higher strength. General Ka expressions take the form Ka = [H3O+][A-] / [HA]. General Kb expressions take the form Kb = [BH+][OH-] / [B].
Q. What is a KB reaction?
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. Similarly, the equilibrium constant for the reaction of a weak base with water is the base ionization constant (Kb). For any conjugate acid–base pair, KaKb=Kw.
Q. How do you find the KB value?
Solve the equation for Kb by dividing the Kw by the Ka. You then obtain the equation Kb = Kw / Ka. Put the values from the problem into the equation. For example, for the chloride ion, Kb = 1.0 x 10^-14 / 1.0 x 10^6.
Q. What is the Ka of HCl?
| Ka | Acid | |
|---|---|---|
| Large | Perchloric acid | HClO4 |
| 3.2 * 109 | Hydroiodic acid | HI |
| 1.0 * 109 | Hydrobromic acid | HBr |
| 1.3 * 106 | Hydrochloric acid | HCl |
Q. How do you calculate ka?
Dissociation Constant for Acetic Acid Since x = [H3O+] and you know the pH of the solution, you can write x = 10-2.4. It is now possible to find a numerical value for Ka. Ka = (10-2.4)2 /(0.9 – 10-2.4) = 1.8 x 10-5.
Q. What is the kb value for H2PO4?
2- is 4.2 x 10-13 and Ka for H2PO4 – is 6.2 x 10-8. Whether this ion will cause the solution to be acidic or basic depends on whether the Ka for reaction A is bigger than the Kb for reaction B.
Q. Is KW a constant?
The equilibrium constant for this reaction is called the ion-product constant of liquid water (Kw) and is defined as Kw=[H3O+][OH−]. At 25 °C, Kw is 1.01×10−14; hence pH+pOH=pKw=14.00.
Q. What is KW equilibrium constant?
Definition of pH and pOH The equilibrium constant, Kw, is called the dissociation constant or ionization constant of water. In pure water [H+] = [OH-] = 1.00×10-7 M. pH and pOH. Working with numbers like 1.00×10-7 M to describe a neutral solution is a rather inconvient.
Q. What is the value of KW at 298 K?
d The value of Kw is 10-14.
Q. Why does KW not include water?
You may wonder why the water isn’t written on the bottom of these equilibrium constant expressions. Kw is defined to avoid making the expression unnecessarily complicated by including another constant in it. The value of Kw. Like any other equilibrium constant, the value of Kw varies with temperature.