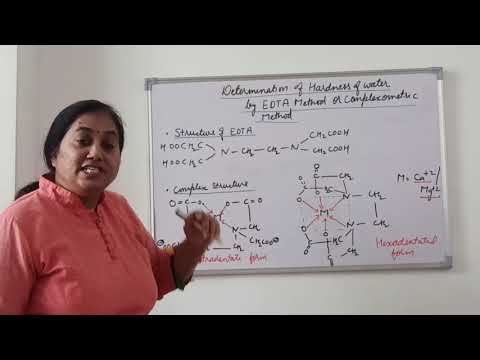
Q. What is full form of EDTA and EBT?
EDTA can form four or six coordination bonds with a metal ion. 1. Total hardness. Total hardness is due to the presence of bicarbonates, chlorides and sulphates of calcium and magnesium ions. The total hardness of water is estimated by titrating the water sample against EDTA using Eriochrome Black-T (EBT) indicator.
Q. What is the chemical name of EBT?
Eriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. in the water hardness determination process. It is an azo dye. Eriochrome is a trademark of Huntsman Petrochemical, LLC. In its deprotonated form, Eriochrome Black T is blue.
Table of Contents
- Q. What is full form of EDTA and EBT?
- Q. What is the chemical name of EBT?
- Q. What is the molecular structure of eriochrome black T?
- Q. What is the Colour of eriochrome black T indicator?
- Q. How do you make Solochrome black indicator?
- Q. Does EDTA bind with EBT?
- Q. What is the Denticity of EBT?
- Q. How many moles of EDTA react with Ca2+?
- Q. How do you standardize EDTA solution?
- Q. Why do we standardize EDTA solution?
- Q. What is the purpose of EDTA standardization?
- Q. How do you make 0.05 N EDTA solution?
- Q. How do you make 0.02 N EDTA solution?
- Q. Why is EDTA not soluble in water?
- Q. What is the relation between molarity and normality?
- Q. What is EDTA powder?
- Q. What is EDTA in Engineering Chemistry?
Q. What is the molecular structure of eriochrome black T?
C20H12N3O7SNa
Q. What is the Colour of eriochrome black T indicator?
blue
Q. How do you make Solochrome black indicator?
Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. Add about 50 mL of 95 percent ethyl alcohol and swirl the mixture until the EBT has fully dissolved.
Q. Does EDTA bind with EBT?
The EDTA-‐Mg mixture will titrate the unknown Ca2+ solution. At the end point, Mg2+ will be released from the EBT indicator and complexed with EDTA, causing the color change from red to blue.
Q. What is the Denticity of EBT?
A solution containing both Mg and Ca is dosed with the complexing agent Erichrome Black T (EBT), which forms a complex with Mg2+. The EBT-Mg complex (K = 10+7) is weaker than the EDTA-Mg complex (K=10+8.7) The color of the EBT-Mg complex is red, and the color of uncomplexedd EBT is blue.
Q. How many moles of EDTA react with Ca2+?
One mole of Ca2+ ions will react with one mole of EDTA ions.
Q. How do you standardize EDTA solution?
Preparation and Standardization of 0.1 M Disodium Edetate (EDTA)
- Take about 100 ml of water in a cleaned and dried 1000 ml volumetric flask.
- Add about 37.2 gm of Disodium Edetate with continues stirring.
- Add more about 700 ml of water mix.
- Make up the volume 1000 ml with water.
Q. Why do we standardize EDTA solution?
Since [Metal ion]+ is unknown, you can make no measurement of the amount present, unless you know [EDTA] fairly accurately. And thus a known mass of primary standard is required to standardize, to calibrate the titration.
Q. What is the purpose of EDTA standardization?
This standardized EDTA solution is then used to determine water “hardness” – the total calcium and magnesium content, typically expressed as equivalent calcium carbonate mass concentration.
Q. How do you make 0.05 N EDTA solution?
Preparation of 0.05 M EDTA Solution Dissolve 18.6 g of disodium ethylenediaminetetraacetate dihydrate (EDTA)(C10H14N2Na2O8·2H2O)13 in sufficient water to make 1000 mL, and store in a polyethylene container.
Q. How do you make 0.02 N EDTA solution?
Mix the disodium EDTA with water, use NaOH to get it to dissolve, and then add distilled water to reach the final volume.
- 0.02 N EDTA. 3.732 g disodium EDTA. 1 liter water. NaOH pellets or solution.
- 0.1 M EDTA. 37.2 g disodium EDTA. 1 liter water. NaOH.
- 100 mL 0.5M EDTA. 18.61 g disodium EDTA. 100 mL water. NaOH.
Q. Why is EDTA not soluble in water?
The carboxyl groups of EDTA are not dissociated at low pH. Undissociated carboxyls (COOH) have no charge because the hydrogen is covalently bound and therefore acid EDTA is almost insoluble in water.
Q. What is the relation between molarity and normality?
There is a very close relation between molarity and normality. Normality can be described as a multiple of molarity. While Molarity refers to the concentration of a compound or ion in a solution, normality refers to the molar concentration only of the acid component or only of the base component of the solution.
Q. What is EDTA powder?
EDTA (ethylenediaminetetraacetic acid) is a chelating agent that binds divalent metal ions such as calcium and magnesium. EDTA can be used to prevent degradation of DNA and RNA and to inactivate nucleases that require metal ions. EDTA can also be used to inactivate metal ion-requiring enzymes.
Q. What is EDTA in Engineering Chemistry?
EDTA stands for Ethylene Diamine Tetra Acetic acid. As it is insoluble in water, we use its disodium salt. When EBT indicator is added to water sample, it forms a wine red coloured unstable Ca-Mg-EBT complex. This reaction is carried out under a basic PH of 8- 10 using ammonia buffer.