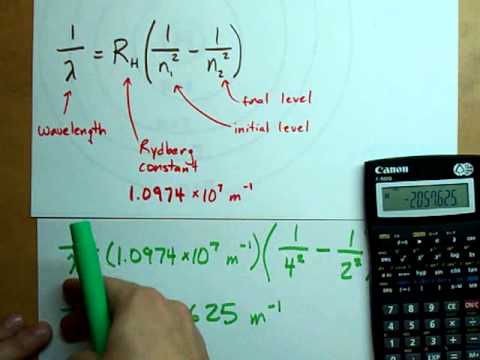
Answer: In a model of a hydrogen atom where n means the energy level (n=1 is the ground state where the electron is closest to the nucleus; n=2 is an excited state where the electron is farther from the nucleus; n=3 is another excited state where the electron is even farther from the nucleus…), you can use the …
Q. How much energy would it take to remove the electron in an H atom if it was in the N 2 level?
Hence, the energy required to remove an electron from $$n=2$$ state in hydrogen is +3.
Table of Contents
- Q. How much energy would it take to remove the electron in an H atom if it was in the N 2 level?
- Q. What energy would be needed to remove the electron from the N 4 level of the hydrogen atom?
- Q. When an electron does transition from N 4 to N 2?
- Q. Which occurs if an electron transitions from N 5 to N 2 in a hydrogen atom?
- Q. Which color of light would a hydrogen atom emit when an electron changes from N 5 to N 2?
- Q. Which element produced the smallest number of lines?
- Q. Why atomic spectra are not continuous?
- Q. How is it possible to identify an element by looking at its spectrum?
- Q. Why do metals have line spectrum?
- Q. How can we use light to identify an element?
Q. What energy would be needed to remove the electron from the N 4 level of the hydrogen atom?
What energy would be needed to remove the electron from the n=4 level of the hydrogen atom? E=13.6 ⋅142eV=0.85eV=1.36⋅10−19J.
Q. When an electron does transition from N 4 to N 2?
Explanation: Jump to second orbit leads to Balmer series. The jump from 4th orbit shall give rise to second line of Balmer series.
Q. Which occurs if an electron transitions from N 5 to N 2 in a hydrogen atom?
Which occurs if an electron transitions from n = 5 to n = 2 in a hydrogen atom? Energy is absorbed, and visible light is emitted.
Q. Which color of light would a hydrogen atom emit when an electron changes from N 5 to N 2?
green
Q. Which element produced the smallest number of lines?
hydrogen
Q. Why atomic spectra are not continuous?
Quick answer: Atomic spectra are continuous because the energy levels of electrons in atoms are quantized. The electrons in an atom can have only certain energy levels. Each packet of energy corresponds to a line in the atomic spectrum. There is nothing between each line, so the spectrum is discontinuous.
Q. How is it possible to identify an element by looking at its spectrum?
By looking at the pattern of lines, scientists can figure out the energy levels of the elements in the sample. Since every element has unique energy levels, the spectra can help identify elements in a sample.
Q. Why do metals have line spectrum?
When atoms are excited they emit light of certain wavelengths which correspond to different colors. Each element produces a unique set of spectral lines. Since no two elements emit the same spectral lines, elements can be identified by their line spectrum.
Q. How can we use light to identify an element?
Each natural element has a characteristic light spectrum that helps identify it in samples of unknown substances. Spectroscopy is the practice of examining spectra and comparing them to those of known elements. Using spectroscopy methods, scientists can identify pure substances or compounds and the elements in them.