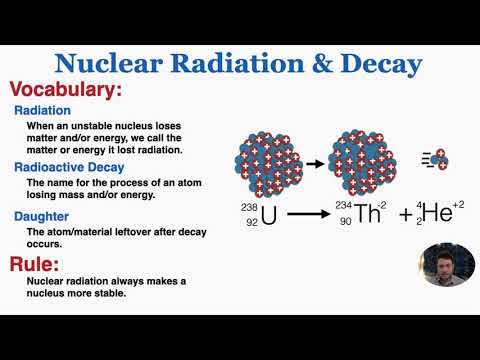
2. Radioactive decay (nuclear decay) Radioactive decay is a process in which an unstable nucleus transforms into a more stable one by releasing particles or photons. Most of this energy is imparted as kinetic energy to released particles or is converted to photons with a small portion as kinetic energy.
Q. What kind of process is radioactive decay according to the clip?
Answer. Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive.
Table of Contents
- Q. What kind of process is radioactive decay according to the clip?
- Q. What happens to a radioisotope when it undergoes nuclear decay?
- Q. How radioactive decay is relevant to our lives?
- Q. What is radioactive decay for dummies?
- Q. Why radiation is so important in our life?
- Q. What is random nature of radioactive decay?
- Q. What is spontaneous decay?
- Q. Are all radioactive decay first order?
- Q. How do you find radioactive decay?
- Q. How do you do radioactive decay in math?
- Q. What is the order of radioactive decay?
- Q. What is N in radioactive decay?
- Q. What is first order decay?
- Q. What is the zero order reaction?
Q. What happens to a radioisotope when it undergoes nuclear decay?
Explanation: When an unstable isotope undergoes radioactive decay radiation is emitted in the form of either alpha, beta, or gamma particles. A change in the number of protons changes the radioactive isotope into a more stable isotope.
Q. How radioactive decay is relevant to our lives?
High frequencies of light can carry enough energy that they can affect the deoxyribonucleic acid (DNA) in your cells, sometimes causing that cell, and its “offspring” to become malignant cancer cells, which can lead to cases of Melanoma among other things.
Q. What is radioactive decay for dummies?
Radioactive decay occurs when an atom loses one or a combination of particles. In the atom’s nucleus, the protons and neutrons give the atomic mass of an atom. Alpha decay occurs with the loss of protons and neutrons, beta decay with the loss of electrons, while gamma decay is an secondary decay energy state change.
Q. Why radiation is so important in our life?
Today, to benefit humankind, radiation is used in medicine, academics, and industry, as well as for generating electricity. In addition, radiation has useful applications in such areas as agriculture, archaeology (carbon dating), space exploration, law enforcement, geology (including mining), and many others.
Q. What is random nature of radioactive decay?
The nuclei of radioactive atoms are unstable. They break down and change into a completely different type of atom. This is called radioactive decay. For example, carbon-14 decays to nitrogen-14 when it emits beta radiation.
Q. What is spontaneous decay?
Spontaneous fission, type of radioactive decay in which certain unstable nuclei of heavier elements split into two nearly equal fragments (nuclei of lighter elements) and liberate a large amount of energy. …
Q. Are all radioactive decay first order?
Because radioactive decay is a first-order process, the time required for half of the nuclei in any sample of a radioactive isotope to decay is a constant, called the half-life of the isotope. Radioactive decay is a first-order process.
Q. How do you find radioactive decay?
- Radioactive decay shows disappearance of a constant fraction of. activity per unit time.
- Half-life: time required to decay a sample to 50% of its initial. activity: 1/2 = e –(λ*T1/2)
- Constant in time, characteristic for each nuclide. Convenient to calculate the decay factor in multiples of T1/2:
Q. How do you do radioactive decay in math?
Mathematically, we represent this as −dNdt=Nλ − d N d t = N λ where dNdt d N d t is the number of decays per second the batch of atoms is undergoing, N is the current number of radioactive atoms, and λ is a constant (called the decay constant) which is characteristic of any particular radioactive atom representing the …
Q. What is the order of radioactive decay?
The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction: t 1/2 = 0.693/k. Radioactive decay reactions are first-order reactions. The rate of decay, or activity, of a sample of a radioactive substance is the decrease in the number of radioactive nuclei per unit time.
Q. What is N in radioactive decay?
Suppose N is the size of a population of radioactive atoms at a given time t, and dN is the amount by which the population decreases in time dt; then the rate of change is given by the equation dN/dt = −λN, where λ is the decay constant. …
Q. What is first order decay?
First order decay simply means that for a population of atoms (e.g. radioactive), molecules (our example of A –> B), or anything else, a constant fraction/unit time is converted to something else. The actual fraction/unit time is expressed as k (the rate constant, in units of time ).
Q. What is the zero order reaction?
: a chemical reaction in which the rate of reaction is constant and independent of the concentration of the reacting substances — compare order of a reaction.