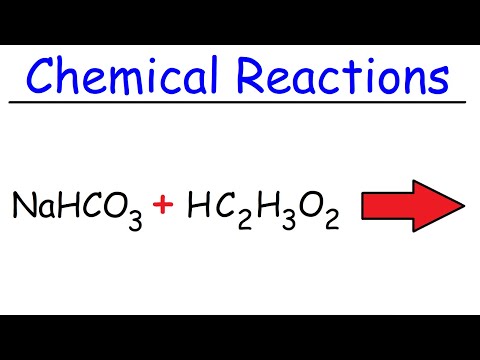
Q. What is the balanced chemical equation for the reaction of vinegar and baking soda?
Here, NaHCO3 is sodium bicarbonate, CH3COOH is acetic acid, CO2 is carbon dioxide, H2O is water, Na+ is the sodium cation, and CH3COO– is the acetate anion. Also, s = solid, l = liquid, g = gas, aq = aqueous or in water solution.
Q. What is the reaction of sodium bicarbonate and vinegar?
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and sodium acetate. The solid baking soda was placed in liquid vinegar producing carbon dioxide gas, which is evident because of the formation of bubbles in the foaming mixture.
Table of Contents
- Q. What is the balanced chemical equation for the reaction of vinegar and baking soda?
- Q. What is the reaction of sodium bicarbonate and vinegar?
- Q. What type of reaction is sodium bicarbonate and acetic acid?
- Q. What type of reaction is sodium bicarbonate?
- Q. What is sodium bicarbonate used for medically?
- Q. What is the formula for vinegar?
- Q. Can baking soda damage kidneys?
- Q. What does baking soda do to the body?
- Q. How long does sodium bicarbonate stay in your system?
- Q. How does sodium bicarbonate help kidneys?
- Q. Is sodium bicarbonate good for kidneys?
- Q. Can you overdose on sodium bicarbonate?
- Q. Can baking soda reverse kidney failure?
- Q. What is the antidote for sodium bicarbonate?
- Q. How much baking soda is safe?
- Q. What does sodium bicarbonate look like?
- Q. Can sodium bicarbonate be given IV push?
- Q. Why is sodium bicarbonate given IV?
- Q. What medications can be given IV push?
- Q. Can sodium bicarbonate be diluted in normal saline?
- Q. How do you dilute sodium bicarbonate?
Q. What type of reaction is sodium bicarbonate and acetic acid?
The carbon dioxide will actually bubble out of solution. This is a classic example of a neutralization reaction in which sodium bicarbonate, or baking soda, is neutralized by a solution of acetic acid, or vinegar.
Q. What type of reaction is sodium bicarbonate?
decomposition reaction
Q. What is sodium bicarbonate used for medically?
Sodium bicarbonate is an antacid used to relieve heartburn and acid indigestion. Your doctor also may prescribe sodium bicarbonate to make your blood or urine less acidic in certain conditions.
Q. What is the formula for vinegar?
CH₃COOH
Q. Can baking soda damage kidneys?
“In fact, in patients taking sodium bicarbonate, the rate of decline in kidney function was similar to the normal age-related decline,” says Yaqoob. Rapid progression of kidney disease occurred in just nine percent of patients taking sodium bicarbonate, compared to 45 percent of the other group.
Q. What does baking soda do to the body?
Sodium bicarbonate is a salt that breaks down to form sodium and bicarbonate in water. This breakdown makes a solution alkaline, meaning it is able to neutralize acid. Because of this, sodium bicarbonate is often used to treat conditions caused by high acidity in the body, such as heartburn.
Q. How long does sodium bicarbonate stay in your system?
Just keep in mind that the effects last only up to 24 hours after the last dose ( 37 , 38 ). Bottom Line: Sodium bicarbonate can be found in powder, pill or capsule form.
Q. How does sodium bicarbonate help kidneys?
Published findings suggest that, for patients with chronic kidney disease, a higher dose of sodium bicarbonate lowered urinary ammonium excretion and increased serum bicarbonate more than a lower dose. However, the higher dose was associated with a greater increase in urinary albumin excretion.
Q. Is sodium bicarbonate good for kidneys?
BUDAPEST, Hungary — Sodium bicarbonate — long used, albeit sporadically, to correct metabolic acidosis in chronic kidney disease — is significantly better at slowing disease progression than standard care, and is safe, results from a large Italian trial indicate.
Q. Can you overdose on sodium bicarbonate?
Overdose of sodium bicarbonate (sodium bicarbonate 5% injection) may result in a severe degree of alkalosis which may be accompanied by hyperirritability or tetany. Should alkalosis result, the bicarbonate should be stopped and the patient managed according to the degree of alkalosis present.
Q. Can baking soda reverse kidney failure?
“A daily dose of baking soda could help patients with chronic kidney disease avoid having to undergo dialysis,” reported The Times . It said that research has found that sodium bicarbonate can dramatically slow the progress of the condition.
Q. What is the antidote for sodium bicarbonate?
Wax, Paul M. “Antidotes in Depth (A5): Sodium Bicarbonate.” Goldfrank’s Toxicologic Emergencies, 9e Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE….Jump to a Section.
| Mechanism | Site of Action | Uses |
|---|---|---|
| Correct life-threatening acidosis | Metabolic | Cyanide Ethylene glycol Methanol Metformin |
Q. How much baking soda is safe?
Baking soda is a good treatment for immediate relief from occasional acid reflux. The recommended dosage for adults is one 1/2 tsp. dissolved in a 4-ounce glass of water. It’s best to sip this drink slowly to avoid side effects like gas and diarrhea.
Q. What does sodium bicarbonate look like?
Sodium bicarbonate is a white solid that is crystalline, but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite.
Q. Can sodium bicarbonate be given IV push?
Sodium Bicarbonate: corrects metabolic acidosis during a cardiac arrest. It is administered by IV push in a dose of 50ml, D5W solution, 44.6mEg of sodium bicarbonate. Metabolic acidosis occurs after the heart stops, due to a buildup of the acid waste materials in the body.
Q. Why is sodium bicarbonate given IV?
Intravenous sodium bicarbonate is indicated in the treatment of metabolic acidosis, such as can occur in severe kidney disease, diabetic ketoacidosis, circulatory insufficiency, extracorporeal circulation of blood, in hemolysis requiring alkalinization of the urine to avoid nephrotoxicity of blood pigments, and certain …
Q. What medications can be given IV push?
Cefazolin, cefotaxime, cefotetan, cefoxitin, ceftazidime, and cefuroxime are FDA-approved for IV push administration.
Q. Can sodium bicarbonate be diluted in normal saline?
Conclusion: When 100 mL of 8.4% sodium bicarbonate are diluted in 150 mL of normal saline within a 250 mL polyolefin bag, changes in pH and Pco2 over a 48-hour period are small and bicarbonate concentration remains stable.
Q. How do you dilute sodium bicarbonate?
Dilute the 8.4% sodium bicarbonate solution with an equal volume of water for injection. The resulting solution contains 0.5mmol/mL sodium bicarbonate. Dose 2mmol 4mmol 6mmol 8mmol 10mmol Volume 4mL 8mL 12mL 16mL 20mL Shake well to ensure thorough mixing. Administered as an infusion over 30 to 60 minutes.