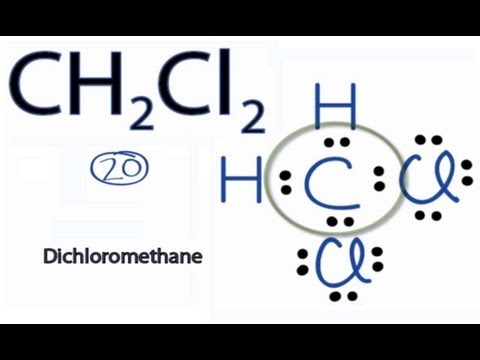
Dichloromethane (DCM or methylene chloride) is an organochloride compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is polar, and miscible with many organic solvents.
Q. What is full form of ch2?
Alternative Title: ethene. Ethylene (H2C=CH2), the simplest of the organic compounds known as alkenes, which contain carbon-carbon double bonds. It is a colourless, flammable gas having a sweet taste and odour.
Table of Contents
- Q. What is full form of ch2?
- Q. Why is ch2cl2 called methylene chloride?
- Q. What is the common name of CH3Cl?
- Q. Is methylene chloride a Geminal Dihalide?
- Q. What is methylene chloride structure?
- Q. What are the uses of methylene chloride?
- Q. Is methylene chloride safe in coffee?
- Q. How do you handle methylene chloride?
Q. Why is ch2cl2 called methylene chloride?
It is named like methylene dichloride(Cl-CH2-Cl) because of having the -CH2- group which is known as methylene group. But its name in IUPAC system is dichloromethane. Here, the structure is not like CHCl rather the carbon atom is bridged between the two Cl-atoms via a -CH.
Q. What is the common name of CH3Cl?
methyl chloride
Q. Is methylene chloride a Geminal Dihalide?
since it is a gem dihalide, so its name should be methyledene and not methylene acc. to the rules… The IUPAC name of the compound CH2Cl2 is Dichloromethane. Its common name is methylene chloride. As a result, while naming this compound we can neither use geminal or vicinal.
Q. What is methylene chloride structure?
CH2Cl2
Q. What are the uses of methylene chloride?
Methylene chloride, also called dichloromethane, is a volatile, colorless liquid with a chloroform-like odor. Methylene chloride is used in various industrial processes, in many different industries including paint stripping, pharmaceutical manufacturing, paint remover manufacturing, and metal cleaning and degreasing.
Q. Is methylene chloride safe in coffee?
The methylene chloride process is thought by some in the coffee industry to maintain coffee flavor better than other processes. Based on extensive research data, the U.S. Food and Drug Administration (FDA) has determined that methylene chloride is safe for use in coffee decaffeination.
Q. How do you handle methylene chloride?
When handling dichloromethane in the workplace, use the following safety precautions:
- Wear protective clothing. Footwear should cover the entire foot.
- Always wear PPE such as chemical splash goggles and safety gloves.
- Work in a well-ventilated area (preferably in an environment with a fume extraction system).