Q. What is the composition of iron in FeI2?
Percent composition by element
| Element | Symbol | Mass Percent |
|---|---|---|
| Iron | Fe | 18.035% |
| Iodine | I | 81.965% |
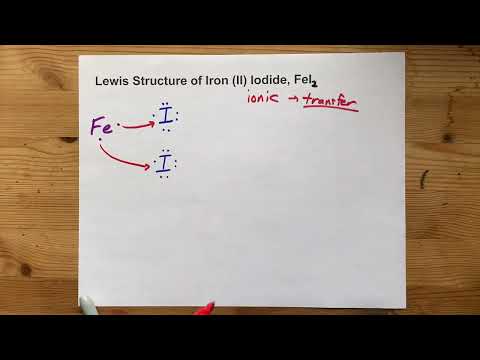
Q. What is the ionic compound fel2?
FeI2 is called Ferrous Iodide or Iron (II) Iodide.
Table of Contents
- Q. What is the composition of iron in FeI2?
- Q. What is the ionic compound fel2?
- Q. Is FeI2 a precipitate?
- Q. Is iron iodide covalent or ionic?
- Q. What is the chemical name for ci4?
- Q. What is the name of pf5?
- Q. Is CI4 a chemical formula?
- Q. Is CI4 tetrahedral?
- Q. Is CI a compound or element?
- Q. Why is CI4 a formula?
- Q. What type of bond is ci4?
- Q. What is ci4 used for?
- Q. Why is iron Ionic?
- Q. What is the ionic charge of an ion with 13 protons and 10 electrons?
- Q. What is the ionic charge of an ion with 13 protons and 11 electrons?
- Q. What is the symbol for an ion having 15 protons and 18 electrons?
- Q. What element has 15 protons and 18 neutrons?
- Q. What has 15 protons and 17 neutrons?
Q. Is FeI2 a precipitate?
1 Expert Answer All species are soluble, so no reaction takes place. No precipitate forms.
Q. Is iron iodide covalent or ionic?
A binary ionic compound is composed of ions of two different elements – one of which is a metal, and the other a nonmetal. For example, iron(III) iodide, FeI3, is composed of iron ions, Fe3+ (elemental iron is a metal), and iodide ions, I- (elemental iodine is a nonmetal).
Q. What is the chemical name for ci4?
CARBON TETRAIODIDE
Q. What is the name of pf5?
PHOSPHORUS PENTAFLUORIDE
Q. Is CI4 a chemical formula?
CI4
Q. Is CI4 tetrahedral?
Carbon tetraiodide is a tetrahalomethane with the molecular formula CI4….Carbon tetraiodide.
| Names | |
|---|---|
| Structure | |
| Crystal structure | Tetragonal |
| Molecular shape | Tetrahedral |
| Dipole moment | 0 D |
Q. Is CI a compound or element?
Chlorine is in group 17 of periodic table, also called the halogens, and is not found as the element in nature – only as a compound.
Q. Why is CI4 a formula?
Carbon tetraiodide is a chemical compound with the formula CI4. This means that one molecule of carbon tetraiodide is formed from one atom of carbon…
Q. What type of bond is ci4?
Iron has an electronegativity of 1.83 and oxygen 3.44. The difference is 3.44 – 1.83 = 1.61. The bond character is polar covalent although the difference is very close to that of ionic bonds. So iron oxide is a polar covalent compound with ionic character.
Q. What is ci4 used for?
It can be used with complete success in over-the-road diesel trucks, off-highway diesel equipment, farm tractors, and passenger cars and light trucks with diesel engines, turbo-charged or non turbo-charged and well as gasoline engines requiring these levels of specifications.
Q. Why is iron Ionic?
In the structure of iron there are positive ions. TRUE. Because the electrons in the outer shell are free to move through the lattice they leave behind positive iron ions.
Q. What is the ionic charge of an ion with 13 protons and 10 electrons?
Answer:Al3+ indicates an ion of aluminum having a charge of + 3. I.e., since an aluminum atom normally has 13 protons and 13 electrons, this ion has 10 electrons (-10 charge) and 13 protons (+ 13 charge) giving it a charge of + 3 (-10 + 13 = +3).
Q. What is the ionic charge of an ion with 13 protons and 11 electrons?
The 13 protons assure us it is aluminum. The difference in protons (13) and electrons (11), tell us that there is an imbalance of 2 positive charges.
Q. What is the symbol for an ion having 15 protons and 18 electrons?
By referring to a periodic table or table of elements, we see that phosphorus (symbol P) has an atomic number of 15. Thus, each atom has 15 protons. The mass number of the ion is 15 + 16 = 31. Because the ion has 15 protons and 18 electrons (three more electrons than protons), its net charge is 3-.
Q. What element has 15 protons and 18 neutrons?
#15 – Phosphorus – P.
Q. What has 15 protons and 17 neutrons?
phosphorus-32