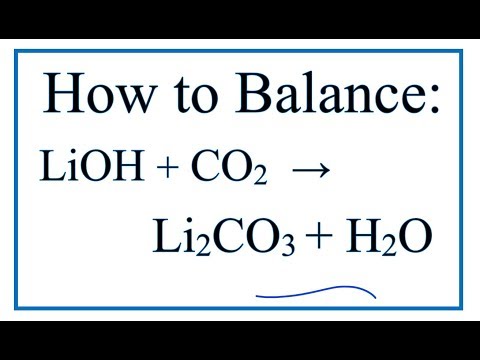
Q. What is the correct balanced equation for the reaction of carbon dioxide and lithium hydroxide to produce lithium carbonate and water?
Problem: Lithium hydroxide absorbs carbon dioxide forming lithium carbonate and water. 2LiOH + CO2 → Li2CO3 + H2OIf a reaction vessel contains 0.15 moles of LiOH and 0.08 moles CO 2, which compound is the limiting reagent?
Q. Which chemical equation is balanced LiOH?
LiOH + CO2 = Li2CO3 + H2O – Chemical Equation Balancer.
Table of Contents
- Q. What is the correct balanced equation for the reaction of carbon dioxide and lithium hydroxide to produce lithium carbonate and water?
- Q. Which chemical equation is balanced LiOH?
- Q. How many grams of lithium hydroxide are needed to completely react with the approximately 900 g of CO2 each astronaut produces each day?
- Q. How does lithium hydroxide remove carbon dioxide?
- Q. What mass in grams of glucose is produced when 3 mol of water react with carbon dioxide?
- Q. What 3 relationships can be derived from a balanced chemical equation?
- Q. What is the chemical equation of mass?
- Q. What are the steps in balancing chemical equation?
- Q. What is the only thing you can change when you balance an equation?
- Q. What are the three example of physical changes?
- Q. What is difference between physical and chemical change?
- Q. Why is cooking of food called a chemical change?
- Q. What chemical reaction happens when you cook an egg?
- Q. Is heating oven a chemical change?
- Q. Is dissolving a physical or chemical change?
- Q. What type of chemical reaction is baking cookies?
- Q. What type of reaction is chemical or physical changes the taste of cookies?
- Q. What does each ingredient do in baking cookies?
- Q. Is dissolving zinc in acid a physical or chemical change?
- Q. Is hydrochloric acid reacts with magnesium to produce hydrogen gas?
Q. How many grams of lithium hydroxide are needed to completely react with the approximately 900 g of CO2 each astronaut produces each day?
About 921 grams of carbon dioxide will react woth 1.00 kg of lithium hydroxide.
Q. How does lithium hydroxide remove carbon dioxide?
The absorption of carbon dioxide is accomplished in a chemical reaction using a sorbent known as lithium hydroxide (LiOH). This method relies on the exothermic reaction of lithium hydroxide with carbon dioxide gas to create lithium carbonate (Li2CO3) solid and water (H2O).
Q. What mass in grams of glucose is produced when 3 mol of water react with carbon dioxide?
What mass, in grams, of glucose is produced when 3.00 mol of water react with carbon dioxide? Answer 10. mol KClO3 1. Ammonia, NH3, is widely used as a fertilizer and in many household cleaners.
Q. What 3 relationships can be derived from a balanced chemical equation?
What relationships can be determined from a balanced chemical equation? The relationships between particles, moles, and mass for all reactants and products. 3. Explain why mole ratios are central to stoichiometric calculations.
Q. What is the chemical equation of mass?
A substance’s molar mass is calculated by multiplying its relative atomic mass by the molar mass constant (1 g/mol). The molar mass constant can be used to convert mass to moles. By multiplying a given mass by the molar mass, the amount of moles of the substance can be calculated.
Q. What are the steps in balancing chemical equation?
How to Balance a Chemical Equation
- Step 1: The Unbalanced Chemical Equation.
- Step 2: Make a List.
- Step 3: Identifying the Atoms in Each Element.
- Step 4: Multiplying the Number of Atoms.
- Step 5: Placing Coefficients in Front of Molecules.
- Step 6: Check Equation.
- Step 7: Balanced Chemical Equation.
- 1 Person Made This Project!
Q. What is the only thing you can change when you balance an equation?
When you balance an equation you can only change the coefficients (the numbers in front of molecules or atoms). Coefficients are the numbers in front of the molecule. Subscripts are the smaller numbers found after atoms. These cannot be changed when balancing chemical equations!
Q. What are the three example of physical changes?
Examples of physical changes are boiling, melting, freezing, and shredding. Many physical changes are reversible, if sufficient energy is supplied. The only way to reverse a chemical change is via another chemical reaction.
Q. What is difference between physical and chemical change?
In a physical change the appearance or form of the matter changes but the kind of matter in the substance does not. However in a chemical change, the kind of matter changes and at least one new substance with new properties is formed. The distinction between physical and chemical change is not clear cut.
Q. Why is cooking of food called a chemical change?
Cooking of food is a chemical change because after cooking, the raw ingredients or the vegetables cannot be regained again.
Q. What chemical reaction happens when you cook an egg?
When you use high heat to boil an egg, it causes a chemical reaction between the yolk and the white that leaves a green film around the yolk. That film is iron sulfide, caused by iron in the yolk reacting with hydrogen sulfide in the white.
Q. Is heating oven a chemical change?
It is considered as a physical change because although it may be difficult, there is a way to separate these ingredients. But when it’s heated in the oven, a chemical reaction occurs and new bonds are formed. It creates chemical reactions.
Q. Is dissolving a physical or chemical change?
To generalize: Dissolving an ionic compound is a chemical change. In contrast, dissolving sugar or another covalent compound is a physical change because chemical bonds are not broken and new products are not formed.
Q. What type of chemical reaction is baking cookies?
When sodium bicarbonate (baking soda) heats up, it causes a chemical reaction: 2NaHCO3 ? Na2CO3 + H2O + CO2. The CO2 gas that’s formed makes the “bubbles” in the cookies.
Q. What type of reaction is chemical or physical changes the taste of cookies?
The reaction is non reversible. The sugar, flour and eggs can no longer be separated. The properties of the materials have changed so it is a chemical change. Baking the cookies is a chemical change, but some of the ingredients may go through a physical change before entering the oven.
Q. What does each ingredient do in baking cookies?
The Science Behind Common Baking Ingredients
- Flour Provides the Recipe Foundation.
- Fat Holds it All Together.
- Sugar Is Sweet and Helps Tenderize.
- Eggs Add Texture.
- Liquids Add Leavening and Tenderness.
- Salt Adds Flavor and Weight.
- Leavening Agents Baking Soda and Baking Powder.
Q. Is dissolving zinc in acid a physical or chemical change?
When zinc reacts with hydrochloric acid, the reaction bubbles vigorously as hydrogen gas is produced (see figure below). The production of a gas is also an indication that a chemical reaction may be occurring. Figure 10.4. 5: Zinc reacts with hydrochloric acid to produce bubbles of hydrogen gas.
Q. Is hydrochloric acid reacts with magnesium to produce hydrogen gas?
Hydrochloric acid reacts with magnesium to produce hydrogen gas.