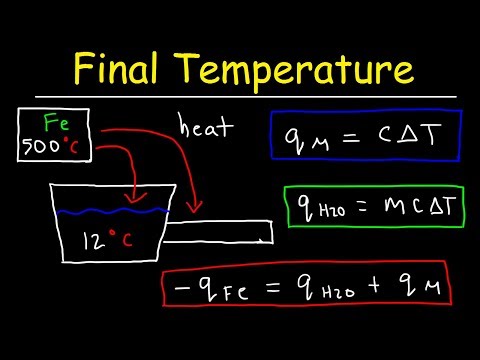
Add the change in temperature to your substance’s original temperature to find its final heat. For example, if your water was initially at 24 degrees Celsius, its final temperature would be: 24 + 6, or 30 degrees Celsius.
Q. How do you calculate the change in liquid temperature?
This is easy. You subtract the final temperature from the starting temperature to find the difference. So if something starts at 50 degrees Celsius and finishes at 75 degrees C, then the change in temperature is 75 degrees C – 50 degrees C = 25 degrees C. For decreases in temperature, the result is negative.
Table of Contents
- Q. How do you calculate the change in liquid temperature?
- Q. What would be the final temperature of the mixture of 50 g?
- Q. When two bodies in contact with each other are at the same temperature they are said to be in?
- Q. What could be the final temperature of a mixture of 100g of water at 90 C and 600g of water at 20 C?
- Q. When the two bodies are in contact with each other?
- Q. What happens when two bodies of different temperature are brought in contact with each other give reason?
- Q. Is it possible for two bodies to be in thermal equilibrium if they are not in contact?
- Q. What is heat balance of human body?
- Q. What is heat balance *?
- Q. What is heat budget or heat balance?
- Q. What is heat balance short answer?
Q. What would be the final temperature of the mixture of 50 g?
Let the specific heat capacity of water be S. Hence, the final temperature of a mixture of 50g of water at 20°C temperature and 50g of water at 40°C temperature would be 30°C.
Q. When two bodies in contact with each other are at the same temperature they are said to be in?
Thermal equilibrium occurs when two bodies are in contact with each other and can freely exchange energy. Systems are in thermal equilibrium when they have the same temperature.
Q. What could be the final temperature of a mixture of 100g of water at 90 C and 600g of water at 20 C?
What could be the final temperature of a mixture of 100 g of water at 90°C and 600g of water at 20°C. (Ans. 30°C)
Q. When the two bodies are in contact with each other?
When the two bodies are placed in contact with each other they will attain thermal equilibrium because the heat flows from higher temperture object to object at lower temperature.
Q. What happens when two bodies of different temperature are brought in contact with each other give reason?
If two bodies at different temperatures are brought together, energy is transferred—i.e., heat flows—from the hotter body to the colder. The effect of this transfer of energy usually, but not always, is an increase in the temperature of the colder body and a decrease in the temperature of the hotter body.
Q. Is it possible for two bodies to be in thermal equilibrium if they are not in contact?
Two bodies are said to be in thermal equilibrium if they are at the same temperature. Therefore, it is possible to have two bodies in thermal equilibrium even though they are not in contact.
Q. What is heat balance of human body?
The core temperature of the body remains steady at around 36.5–37.5 °C (or 97.7–99.5 °F). In the process of ATP production by cells throughout the body, approximately 60 percent of the energy produced is in the form of heat used to maintain body temperature. Thermoregulation is an example of negative feedback.
Q. What is heat balance *?
Definition of heat balance. : the distribution of the heat energy supplied to a thermomechanical system (as a steam power plant) among the various drains upon it including both useful output and losses also : an evaluation or record of such distribution.
Q. What is heat budget or heat balance?
A heat budget is the perfect balance between incoming heat absorbed by earth and outgoing heat escaping it in the form of radiation.
Q. What is heat balance short answer?
Heat balance is defined as the state of equilibrium that exists on earth between incoming insolation from the sun and the out going terrestrial radiation from the earth.