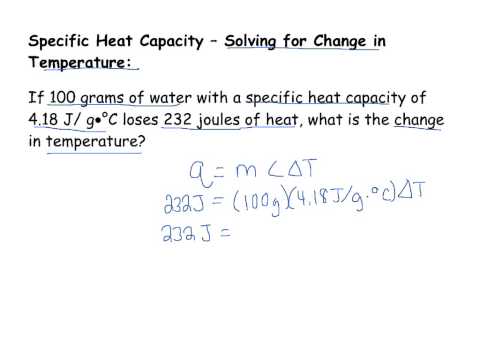Q. What is the formula for calculating change in temperature?
When heat transfer is involved, use this formula: change in temperature = Q / cm to calculate the change in temperature from a specific amount of heat added. Q represents the heat added, c is the specific heat capacity of the substance you’re heating, and m is the mass of the substance you’re heating.
Q. Can the change in temperature be negative?
An exothermic reaction occurs when the temperature of a system increases due to the evolution of heat. This heat is released into the surroundings, resulting in an overall negative quantity for the heat of reaction (qrxn<0).
Table of Contents
- Q. What is the formula for calculating change in temperature?
- Q. Can the change in temperature be negative?
- Q. How do you calculate heat change in joules?
- Q. What is Joule formula?
- Q. How many joules did the water absorb?
- Q. What is the final temperature after 840 joules is absorbed?
- Q. How many joules did the metal lose?
- Q. How many joules of heat energy are released when 50 grams of water are cooled from 70 ºC to 60 ºC?
- Q. How many joules are required to change the temperature of 50.0 g?
- Q. What is the total amount of heat absorbed by 100.0 grams of water when the temperature?
- Q. What is the amount of heat absorbed when the temperature of 75 grams of water?
- Q. How do you calculate the amount of heat absorbed?
- Q. How do you calculate heat absorbed by water?
- Q. What is heat absorption?
- Q. How do you know if heat is absorbed or released?
- Q. What material can absorb the most heat?
- Q. What material absorbs the least amount of heat?
- Q. Does black plastic absorb heat?
- Q. What absorbs the most sunlight?
Q. How do you calculate heat change in joules?
Multiply the change in temperature by the specific heat capacity and the mass of your object. This will give you the heat lost or gained in joules. Example: If 10 kilograms of water are heated from 10 degrees Celsius to 50 degrees Celsius, how much energy (in joules) did they absorb?
Q. What is Joule formula?
In equation form: work (joules) = force (newtons) x distance (meters), One joule is defined as the amount of work done when a force of one newton is exerted through a distance of one meter.
Q. How many joules did the water absorb?
Quantitative experiments show that 4.18 Joules of heat energy are required to raise the temperature of 1g of water by 1°C. Thus, a liter (1000g) of water that increased from 24 to 25°C has absorbed 4.18 J/g°C x 1000g x 1°C or 4180 Joules of energy.
Q. What is the final temperature after 840 joules is absorbed?
The final temperature of 10 gram of water which absorbed 840 joules of energy at an initial temperature of 25 degrees and final temperature of 45 degrees.
Q. How many joules did the metal lose?
1672 joules
Q. How many joules of heat energy are released when 50 grams of water are cooled from 70 ºC to 60 ºC?
1 Answer. 2000 J of heat energy are released.
Q. How many joules are required to change the temperature of 50.0 g?
The answer is 153.7kJ .
Q. What is the total amount of heat absorbed by 100.0 grams of water when the temperature?
6279 Joules
Q. What is the amount of heat absorbed when the temperature of 75 grams of water?
Answer: 4.704625 kJ (or 4704.625 J) is the amount of heat absorbed when the temperature of 75 grams of water increases from 20.
Q. How do you calculate the amount of heat absorbed?
You can do this easily: just multiply the heat capacity of the substance you’re heating by the mass of the substance and the change in temperature to find the heat absorbed.
Q. How do you calculate heat absorbed by water?
The heat absorbed is calculated by using the specific heat of water and the equation ΔH=cp×m×ΔT. 4. Water is vaporized to steam at 100oC. The heat absorbed is calculated by multiplying the moles of water by the molar heat of vaporization.
Q. What is heat absorption?
absorbing heat without increase in temperature when heated beyond a certain point. see more. Antonyms: exothermal, exothermic, heat-releasing. (of a chemical reaction or compound) occurring or formed with the liberation of heat.
Q. How do you know if heat is absorbed or released?
Enthalpy of a reaction is defined as the heat energy change ( Δ H ΔH ΔH ) that takes place when reactants go to products. If heat is absorbed during the reaction, Δ H ΔH ΔH is positive; if heat is released, then Δ H ΔH ΔH is negative.
Q. What material can absorb the most heat?
Zeolite thermal storage retains heat indefinitely, absorbs four times more heat than water.
Q. What material absorbs the least amount of heat?
Three factors determine how good a material is at absorbing and storing heat. The ideal material is: dense and heavy, so it can absorb and store significant amounts of heat (lighter materials, such as wood, absorb less heat)
Q. Does black plastic absorb heat?
White reflects most of the energy falling from the visible spectrum, black absorbs it. When the energy of light is absorbed it turns into heat . Any material painted black will absorb this heat further and its temperature will be raised but it will depend on the material how far the heat is transferred.
Q. What absorbs the most sunlight?
Darker colors tend to absorb more energy from the sun than objects with lighter colors. Someone wearing a white T-shirt in the summer will find that he is cooler than someone wearing a black or dark-colored shirt. This is true of all materials which have dark colors.