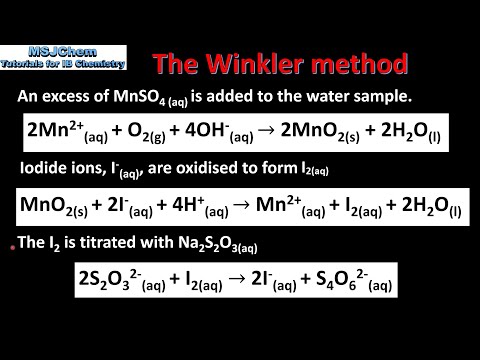
Q. What is the formula for dissolved oxygen?
Rs = R / M, where M is the molecular weight of the gas. Example reading:1 Pa = 1 N/m = 10 bar = 10.197×10 at = 9.8692×10 atm, etc. Less oxygen dissolves at high elevations (Mount Everest)compared to low elevations (sea level) because the atmospheric pressure is less and thus the partial pressure is lower.
Q. Is Dissolved oxygen harmful to fish?
Dissolved oxygen concentrations below 5 mg/L may be harmful to fish and piping (gulping air at the surface) may be observed when DO falls below 2 mg/L. Emergency aeration should be supplied whenever DO falls below 4 mg/L or environmental conditions favor an oxygen depletion event.
Table of Contents
- Q. What is the formula for dissolved oxygen?
- Q. Is Dissolved oxygen harmful to fish?
- Q. Can water absorb oxygen?
- Q. Why is dissolved oxygen bad?
- Q. Is oxygen more important than water?
- Q. Can water have no oxygen?
- Q. What increases dissolved oxygen in water?
- Q. How do you test for dissolved oxygen in water?
- Q. How do humans affect dissolved oxygen in water?
- Q. Does pH affect dissolved oxygen levels in water?
- Q. How does pH impact dissolved oxygen?
Q. Can water absorb oxygen?
Oxygen is absorbed in water by direct diffusion and by surface-water agitation. Solubility of oxygen in water is so small and by diffusion process alone in still water, it was culculated that it would take 6 years for oxygen to diffuse from surface to a depth of 6 meters in quiet water.
Q. Why is dissolved oxygen bad?
Adequate dissolved oxygen is necessary for good water quality. As dissolved oxygen levels in water drop below 5.0 mg/l, aquatic life is put under stress. The lower the concentration, the greater the stress. Oxygen levels that remain below 1-2 mg/l for a few hours can result in large fish kills.
Q. Is oxygen more important than water?
Adequate dissolved oxygen is necessary for good water quality. Oxygen is a necessary element to all forms of life. Biologically speaking, however, the level of oxygen is a much more important measure of water quality than feacal coliform.
Q. Can water have no oxygen?
Anoxic waters are areas of sea water, fresh water, or groundwater that are depleted of dissolved oxygen and are a more severe condition of hypoxia. The US Geological Survey defines anoxic groundwater as those with dissolved oxygen concentration of less than 0.5 milligrams per litre.
Q. What increases dissolved oxygen in water?
Dissolved oxygen levels are increased by supplementing wind and wave action, adding plants to water and exposing water to purified oxygen.
Q. How do you test for dissolved oxygen in water?
Dissolved oxygen levels can be measured by a basic chemical analysis method (titration method), an electrochemical analysis method (diaphragm electrode method), and a photochemical analysis method (fluorescence method). The diaphragm electrode method is the most widely used method.
Q. How do humans affect dissolved oxygen in water?
How do human activities affect the dissolved oxygen concentrations in water? Removal of the trees and plants that grow along the edge of streams and rivers decreases shading, result- ing in warmer water temperatures. This can indirectly cause lower dissolved oxygen concentrations be- cause warm water holds less oxygen.
Q. Does pH affect dissolved oxygen levels in water?
We hypothesize that the dissolved oxygen levels decrease due to increasing levels of pH, thus inhibiting aquatic life that keeps dissolved oxygen levels high.
Q. How does pH impact dissolved oxygen?
A minor increase in pH levels can cause a oligotrophic (rich in dissolved oxygen) lake to become eutrophic (lacking dissolved oxygen). Even minor pH changes can have long-term effects.