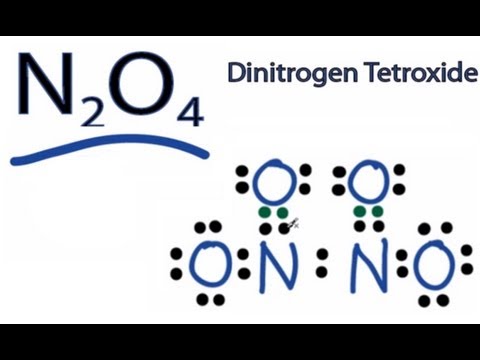
Q. What is the Lewis dot structure of N2O4?
Dinitrogen tetroxide is a one of the oxide of nitrogen and are two nitrogen atoms are located at center of the molecule. In Lewis Structure of N2O4, two oxygen atoms have connected to one nitrogen atom. There are charges on atoms in Lewis Structure of N2O4.
Q. What is the molecular shape of N2O4?
Identify the molecular geometry of N2O4. The molecular geometry is trigonal planar.
Table of Contents
- Q. What is the Lewis dot structure of N2O4?
- Q. What is the molecular shape of N2O4?
- Q. How many valence electrons are in the Lewis structure of N2O4?
- Q. What is the hybridization of N2O4?
- Q. Does N3 exist?
- Q. How many lone pairs does N3 have?
- Q. Does N3 have lone pairs?
- Q. What is the hybridisation of N3?
- Q. How many point groups are there?
- Q. What is the systematic name for N3 -?
- Q. What is the name of ca2+?
- Q. What does Ca ++ stand for?
- Q. What does the superscript 2+ mean in ca2+?
- Q. Is it +2 or 2+ chemistry?
- Q. What is the formula of beryllium?
- Q. Is Al positive or negative?
- Q. Why does Al have a +3 charge?
- Q. What charge does Al have?
- Q. How do you know if a compound is positive or negative?
- Q. How do you determine positive and negative charges?
Q. How many valence electrons are in the Lewis structure of N2O4?
34 valence electrons
Q. What is the hybridization of N2O4?
The hybridization is sp2 which is characteristic of trigonal planar geometry. III. How many sigma bonds and pi bonds are in N2O4? There is one sigma bond between N and N, two sigma bonds between N and O and there are two pi bonds between N and O.
Q. Does N3 exist?
In the Lewis Structure for N3- you’ll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. There are 16 valence electrons for the Lewis structure for N3-.
Q. How many lone pairs does N3 have?
two lone pairs
Q. Does N3 have lone pairs?
In lewis structure of N3- ion contains two N=N bonds. Each outside nitrogen atoms have two lone pairs and center nitrogen atom does not have lone pairs. There are charges on all nitrogen atoms.
Q. What is the hybridisation of N3?
The central N ion forms double bonds with the other 2 N atoms. Thus it has 2 bonding domains and no lone pair of electrons. This gives it a hybridisation of: sp. So, the hybridisation of the central atom of N3− is sp.
Q. How many point groups are there?
In the classification of crystals, each point group defines a so-called (geometric) crystal class. There are infinitely many three-dimensional point groups. However, the crystallographic restriction on the general point groups results in there being only 32 crystallographic point groups.
Q. What is the systematic name for N3 -?
The correct name for the N3 ion is azide. Azide is a polyatomic anion with a -1 charge and is written as N3 -1. It consists of three nitrogen atoms…
Q. What is the name of ca2+?
Calcium ion
Q. What does Ca ++ stand for?
calcium
Q. What does the superscript 2+ mean in ca2+?
If a charge is present, it’s indicated in superscript, with a sign (+/-) and a number if more than one charge is present. For example, calcium ions have two positive charges so are written Ca2+.
Q. Is it +2 or 2+ chemistry?
It is the same convention as when there is multiple of an entity in algebra. If you had two a’s, that is a+a, it is written as 2a, not a2.
Q. What is the formula of beryllium?
Beryllium(2+)
| PubChem CID | 107649 |
|---|---|
| Structure | Find Similar Structures |
| Molecular Formula | Be+2 |
| Synonyms | beryllium(2+) Be(2+) 22537-20-8 beryllium(2+) ion Beryllium element More… |
| Molecular Weight | 9.012183 |
Q. Is Al positive or negative?
Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. Aluminum, a member of the IIIA family, loses three electrons to form a 3+ cation. The halogens (VIIA elements) all have seven valence electrons.
Q. Why does Al have a +3 charge?
The charge of an aluminum ion is typically 3+. This is because the element’s atomic number is 13, reflecting the fact that it has 13 electrons and 13 protons. The valence shell of aluminum has three electrons, and per the octet rule, these three electrons are lost resulting in just 10 electrons and 13 protons.
Q. What charge does Al have?
Table of Common Element Charges
| Number | Element | Charge |
|---|---|---|
| 10 | neon | 0 |
| 11 | sodium | 1+ |
| 12 | magnesium | 2+ |
| 13 | aluminum | 3+ |
Q. How do you know if a compound is positive or negative?
If you look at the periodic table, you might notice that elements on the left side usually become positively charged ions (cations) and elements on the right side get a negative charge (anions). That trend means that the left side has a positive valence and the right side has a negative valence.
Q. How do you determine positive and negative charges?
4 Answers. In order to tell the sign of an object charge, you need another object with a known positive or negative charge. If you rub a piece of glass with silk, it will have a positive charge (by convention). If you rub a piece of amber with fur, it will have a negative charge (also by convention).