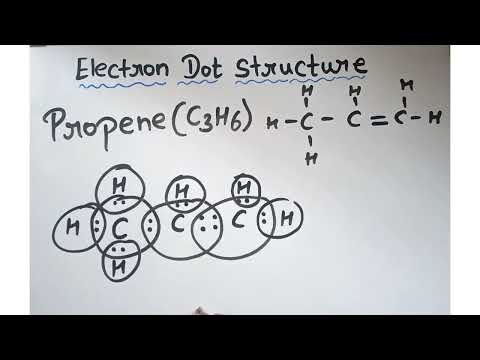
Q. What is the Lewis structure for propene?
VSEPR calculation for propene, CH3CH=CH 2
| Lewis structure: | |
| Central atom: | Carbon (MeCH=CH2) |
| Valence electrons on central atom: | 4 |
| 1 Me group contributes 1 electron: | 1 |
| Total | 6 |
|---|
Q. How do you draw the electron dot structure for propene?
The first is a ring called cyclopropane. It is also possible to draw three carbons in a line with a double bond. Both Lewis structures are correct for C3H6. After determining how many valence electrons there are in C3H6, place them around the central atom to complete the octets.
Table of Contents
- Q. What is the Lewis structure for propene?
- Q. How do you draw the electron dot structure for propene?
- Q. What is the Lewis dot structure for F2?
- Q. What is the formula for Propyne?
- Q. What is alkyne formula?
- Q. What are the first 4 alkanes?
- Q. How alkanes are formed?
- Q. Why are alkanes called paraffins?
- Q. Are alkanes flammable?
- Q. How do alkanes react?
- Q. What are the 3 types of alkane reactions?
- Q. How do alkenes react?
- Q. Why do alkanes not react with potassium permanganate?
- Q. Does cyclohexane react with potassium permanganate?
Q. What is the Lewis dot structure for F2?
The diatomic fluorine molecule (F2) contains a single shared pair of electrons. Each F atom also has three pair of electrons that are not shared with the other atom. A lone pair is a pair of electrons in a Lewis electron-dot structure that is not shared between atoms.
Q. What is the formula for Propyne?
C3H4
Q. What is alkyne formula?
The general formula for alkynes is CnH2n-2. Acetylene is the simplest alkyne with the formula as C2H2.
Q. What are the first 4 alkanes?
The first four alkanes are methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10).
Q. How alkanes are formed?
Alkane can be prepared from alkene and alkyne through the process of hydrogenation. In this process, dihydrogen gas is added to alkynes and alkenes in the present catalyst. This catalysts which are finely divided is like nickel, palladium or platinum to form alkanes.
Q. Why are alkanes called paraffins?
Paraffin is a truncation of Latin ‘parum affinum’ meaning ‘less affinity’ i.e. ‘less reactivity’. Hence, alkanes are called paraffins because they have lesser affinity towards general reagents. In other words they are inert, not readily active.
Q. Are alkanes flammable?
In general, alkanes show a relatively low reactivity. Lower alkanes in particular are highly flammable and form explosive mixtures (methane, benzene) with air (oxygen). Solubility of alkanes in water is very low.
Q. How do alkanes react?
Alkanes undergo a substitution reaction with halogens in the presence of light. For instance, in ultraviolet light , methane reacts with halogen molecules such as chlorine and bromine. This reaction is a substitution reaction because one of the hydrogen atoms from the methane is replaced by a bromine atom.
Q. What are the 3 types of alkane reactions?
Combustion Reactions – burn them – destroying the entire molecule; Halogenation Reactions (substitution type) – react them with some of the halogens, breaking the carbon-hydrogen bonds; Cracking Reactions – use heat and/or a catalyst to crack alkanes, breaking carbon-carbon bonds.
Q. How do alkenes react?
Alkenes react in many addition reactions, which occur by opening up the double-bond. Examples are hydrohalogenation, halogenation, halohydrin formation, oxymercuration, hydroboration, dichlorocarbene addition, Simmons–Smith reaction, catalytic hydrogenation, epoxidation, radical polymerization and hydroxylation.
Q. Why do alkanes not react with potassium permanganate?
Hydrocarbons with only single bonds are called alkanes. Alkanes are called saturated hydrocarbons because each carbon is bonded with as many hydrogen atoms as possible. Potassium permanganate will not react with alkanes since they are saturated.
Q. Does cyclohexane react with potassium permanganate?
Cyclohexane, cyclohexene, and cyclohexanol are added to potassium permanganate solution. Cyclohexane does not react. The secondary alcohol cyclohexanol is oxidized by permanganate to give the ketone cyclohexanone. The alkene cyclohexene reacts to give cis-1,2-cyclohenanediol.