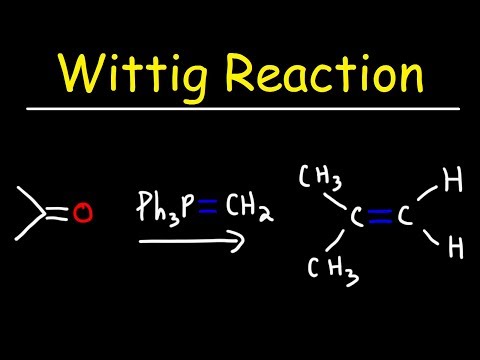
Q. What is the mechanism for the Wittig reaction?
Mechanism of the Wittig Reaction. (2+2) Cycloaddition of the ylide to the carbonyl forms a four-membered cyclic intermediate, an oxaphosphetane. Preliminary posultated mechanisms lead first to a betaine as a zwitterionic intermediate, which would then close to the oxaphosphetane.
Q. How do you make a Wittig reagent?
Wittig reagents are usually prepared from a phosphonium salt, which is in turn prepared by the reaction of triphenylphosphine with an alkyl halide via an SN2 reaction. The alkylphosphonium salt is deprotonated with a strong base such as n-butyllithium: [Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H.
Table of Contents
- Q. What is the mechanism for the Wittig reaction?
- Q. How do you make a Wittig reagent?
- Q. How are ylides made?
- Q. What is the major product of the Wittig reaction?
- Q. Is the Wittig reaction a elimination reaction?
- Q. Which one is the Wittig reagent?
- Q. Which substrate is the best one to use in the formation of the Wittig reagent to make the product displayed?
- Q. What is the limiting reagent in a Wittig Reaction?
- Q. Why is sodium hydroxide used in Wittig reaction?
- Q. What kind of reagent is used in the Wittig reaction?
- Q. Is the Wittig reaction a stepwise mechanism?
- Q. What is the result of the Wittig olefination?
- Q. Are there any limitations to the Wittig reaction?
Q. How are ylides made?
Ylides can be synthesized from an alkyl halide and a trialkyl phosphine. Typically triphenyl phosphine is used to synthesize ylides. Because a SN2 reaction is used in the ylide synthesis methyl and primary halides perform the best. Secondary halides can also be used but the yields are generally lower.
Q. What is the major product of the Wittig reaction?
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene and triphenylphosphine oxide.
Q. Is the Wittig reaction a elimination reaction?
Let’s now discuss the mechanism of the Wittig reaction. It is a nucleophilic addition-elimination reaction and, in that sense, is still somewhat like the other reactions of aldehydes and ketones such as the ones with cyanides, alcohols or amines.
Q. Which one is the Wittig reagent?
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent….
| Wittig reaction | |
|---|---|
| aldehyde or ketone + triphenyl phosphonium ylide ↓ alkene + triphenylphosphine oxide | |
| Conditions | |
| Typical solvents | typically THF or diethyl ether |
Q. Which substrate is the best one to use in the formation of the Wittig reagent to make the product displayed?
[When planning a Wittig, it’s generally best to use a primary alkyl halide (or alkyl sulfonate) here, as secondary alkyl halides don’t work as well. ] The C-H bond adjacent to the phosphorus is relatively acidic [Note 2] and can be deprotonated with strong base to give the ylide shown.
Q. What is the limiting reagent in a Wittig Reaction?
The limiting reagent in this reaction is triphenylphosphine. The product formed appeared a pale brown-orange color; this is an expected property of the desired product.
Q. Why is sodium hydroxide used in Wittig reaction?
In the second step, a base, such as sodium hydroxide (NaOH) or butyl lithium (BuLi), is used to deprotonate and forms the ylide (Wittig reagent). The ylide is used in the Wittig reaction. The ylide then acts as a nucleophile and adds to the carbonyl carbon. The overall reaction is given below.
Q. What kind of reagent is used in the Wittig reaction?
Wittig reaction is a chemical reaction that converts primary or secondary aldehyde, ketone or alkyl halide using a Wittig reagent namely triphenylphosphine to provide triphenylphosphine oxide and alkane.
Q. Is the Wittig reaction a stepwise mechanism?
This intermediate is not very stable and undergoes an intramolecular elimination to give the alkene and triphenylphosphine oxide as a by-product. Depending on your instructor, it might be acceptable to show the Wittig reaction as a stepwise mechanism which helps to show how the bonds are made.
Q. What is the result of the Wittig olefination?
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene and triphenylphosphine oxide. The Wittig reaction was discovered in 1954 by Georg Wittig, for which he was awarded the Nobel Prize in Chemistry in 1979.
Q. Are there any limitations to the Wittig reaction?
The geometry of the double bond can easily be predicted if the Ylide’s nature is known. A few limitations of the Wittig reaction are: Both the E and the Z double bond isomers can be formed. The reaction speed is very slow when sterically hindered ketones are used. The yield is also low for these reactions.