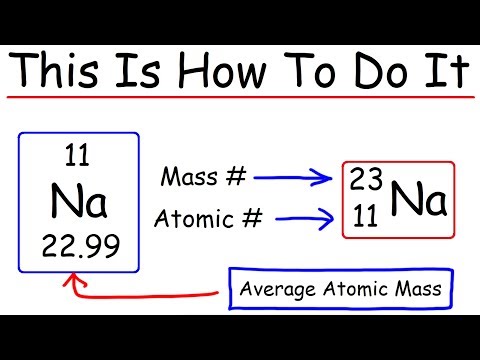
Q. What is the name of an atom with an atomic mass number of 18 and 8 protons?
Isotopes are forms of a chemical element that have the same atomic number but differ in mass. 16O → 8 protons + 8 neutrons; a “light” oxygen); The relative amounts are expressed as either 18O/16O or δ 18O Oxygen – 18 (aka 18O → 8 protons + 10 neutrons; a “heavy” oxygen).
Q. What will be the mass number of oxygen if its atomic number is 8 and number of neutrons is 8?
The atomic number of oxygen is 8. The mass of an atom is given by the sum of the masses of protons and neutrons present in the nucleus. Also, atomic number is the number of protons present in the nucleus. Since, the number of neutrons present in oxygen is 8 and given mass is 16.
Table of Contents
- Q. What is the name of an atom with an atomic mass number of 18 and 8 protons?
- Q. What will be the mass number of oxygen if its atomic number is 8 and number of neutrons is 8?
- Q. How many neutrons does oxygen atom have if it has an atomic number of 8 and an atomic mass of 16?
- Q. How many electrons are in an oxygen atom if its atomic number is 8 and its charge is?
- Q. How many electrons are in an atom of oxygen?
- Q. How many protons are in an atom of oxygen?
- Q. Why is oxygen electrically neutral?
- Q. How many electrons are in a neutral atom of oxygen?
- Q. How many electrons does a neutral atom of oxygen 18 have?
- Q. What is the mass of oxygen 16 atom?
- Q. How many electrons does oxygen 16 have?
- Q. How many mass number does oxygen have?
- Q. How do you know how many electrons are in oxygen?
- Q. How many stable electrons does oxygen have?
- Q. How many electrons are in the outer shell of S?
- Q. How do you find number of electrons?
- Q. How many electrons are there in lithium?
- Q. Can lithium have 2 electrons?
Q. How many neutrons does oxygen atom have if it has an atomic number of 8 and an atomic mass of 16?
Because protons and neutrons are roughly equal in mass, an isotope’s number is equal to the sum of its protons and neutrons. Therefore, oxygen 16 has 8 protons and 8 neutrons, oxygen 17 has 8 protons and 9 neutrons, and oxygen 18 has 8 protons and 10 neutrons.
Q. How many electrons are in an oxygen atom if its atomic number is 8 and its charge is?
For example: O2- indicates an ion of oxygen having two extra electrons. I.e., since an oxygen atom normally has 8 protons and 8 electrons, this ion has 10 electrons (-10 charge) and 8 protons (+8 charge) giving it a charge of -2 (-10 + 8 = -2).
Q. How many electrons are in an atom of oxygen?
2, 6
Q. How many protons are in an atom of oxygen?
8
Q. Why is oxygen electrically neutral?
Explanation: For oxygen, Z , the atomic number =8 . There are 8 protons in its nucleus (and protons are positively charged particles). To balance this charge (the atom is electrically neutral after all), the oxygen nucleus is surrounded by 8 negatively charged electrons.
Q. How many electrons are in a neutral atom of oxygen?
8 electrons
Q. How many electrons does a neutral atom of oxygen 18 have?
Q. What is the mass of oxygen 16 atom?
15.994914
Q. How many electrons does oxygen 16 have?
Q. How many mass number does oxygen have?
16
Q. How do you know how many electrons are in oxygen?
Q. How many stable electrons does oxygen have?
2 electrons
Q. How many electrons are in the outer shell of S?
6 electrons
Q. How do you find number of electrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
Q. How many electrons are there in lithium?
2,1
Q. Can lithium have 2 electrons?
So… for the element of LITHIUM, you already know that the atomic number tells you the number of electrons. That means there are 3 electrons in a lithium atom. Looking at the picture, you can see there are two electrons in shell one and only one in shell two.