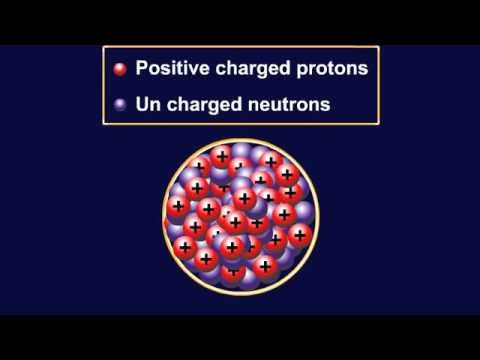
Q. What is the positively charged nucleus composed of?
protons
Q. What is found at the center of an atom?
The parts of an atom are protons, electrons, and neutrons. A proton is positively charged and is located in the center or nucleus of the atom. Electrons are negatively charged and are located in rings or orbits spinning around the nucleus. The number of protons and electrons is always equal.
Table of Contents
- Q. What is the positively charged nucleus composed of?
- Q. What is found at the center of an atom?
- Q. What is the charge of the center of an atom?
- Q. What is an atom diagram?
- Q. What is Atom and example?
- Q. Is there a picture of an atom?
- Q. How do we know the structure of an atom?
- Q. Why is it important to know the structure of an atom?
- Q. Who discovered the basic structure of an atom?
- Q. Which atom is the smallest?
- Q. How does an atom look like?
- Q. Can we see an atom yet?
- Q. What is inside the quark?
Q. What is the charge of the center of an atom?
The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
Q. What is an atom diagram?
Atomic diagrams were developed to explain the interaction of the elements of the Earth and space long before atoms could be observed. Nowadays, scientists can see particles that are smaller than an atom. These sub-atomic particles are the basis of particle physics.
Q. What is Atom and example?
Many atoms consist of a positively charged nucleus consisting of protons and neutrons surrounded by a cloud of electrons charged negatively. An atom is any particle of matter at its most basic level which contains at least one proton. Here are some examples of the atoms: hydrogen (H) neon (Ne).
Q. Is there a picture of an atom?
It’s tiny, but it’s visible. Atoms are so small that it’s almost impossible to see them without microscopes. But now, an award-winning photo shows a single atom in an electric field—and you can see it with your naked eye if you really look hard.
Q. How do we know the structure of an atom?
There are three ways that scientists have proved that these sub-atomic particles exist. They are direct observation, indirect observation or inferred presence and predictions from theory or conjecture.
Q. Why is it important to know the structure of an atom?
4.4 Structure of the atom (ESAAZ) As a result of the work done by previous scientists on atomic models, scientists now have a good idea of what an atom looks like. This knowledge is important because it helps us to understand why materials have different properties and why some materials bond with others.
Q. Who discovered the basic structure of an atom?
John Dalton
Q. Which atom is the smallest?
helium
Q. How does an atom look like?
99.9999999999999% of an atom is actually empty space. Physicists don’t think of the nucleus as a cluster of protons and neutrons like a cluster of grapes. They actually learn about it in a similar way as the electron. They actually shoot electrons into the nucleus and protons and neutrons come out the other side.
Q. Can we see an atom yet?
Atoms are extremely small measuring about 1 x 10-10 meters in diameter. Because of their small size, it’s impossible to view them using a light microscope. While it may not be possible to view an atom using a light microscope, a number of techniques have been developed to observe and study the structure of atoms.
Q. What is inside the quark?
Quark. A proton is composed of two up quarks, one down quark, and the gluons that mediate the forces “binding” them together. The color assignment of individual quarks is arbitrary, but all three colors must be present; red, blue and green are used as an analogy to the primary colors that together produce a white color …