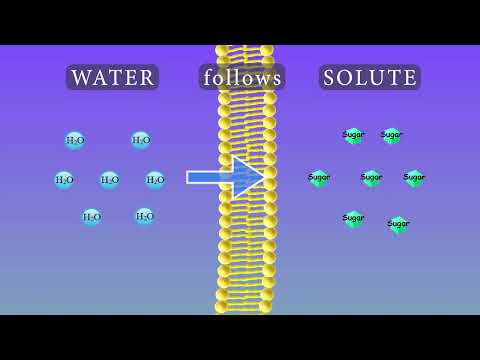
Q. What is the process of osmosis?
Osmosis, the spontaneous passage or diffusion of water or other solvents through a semipermeable membrane (one that blocks the passage of dissolved substances—i.e., solutes). The process, important in biology, was first thoroughly studied in 1877 by a German plant physiologist, Wilhelm Pfeffer.
Q. Do cells lose water in a hypertonic solution?
Hypertonic solutions have less water ( and more solute such as salt or sugar ) than a cell. If you place an animal or a plant cell in a hypertonic solution, the cell shrinks, because it loses water ( water moves from a higher concentration inside the cell to a lower concentration outside ).
Table of Contents
- Q. What is the process of osmosis?
- Q. Do cells lose water in a hypertonic solution?
- Q. What is it called when a cell loses water and shrinks?
- Q. What is hypotonic and hypertonic solution?
- Q. How is isotonic defined?
- Q. What is isotonic to blood?
- Q. What is the isotonic value?
- Q. How is isotonic value calculated?
- Q. What is the isotonic point of a potato?
- Q. Which one is the same in isotonic solution?
- Q. What are normal solutions?
- Q. What is unit of molality?
- Q. Do isotonic solutions have same density?
- Q. What is molarity of pure water?
- Q. Is blood plasma isotonic?
- Q. Is isotonic solutions have same boiling point?
- Q. Which is the highest boiling point?
- Q. Which is not a Colligative property?
- Q. What is meant by Colligative property?
- Q. What are the 4 Colligative properties?
- Q. What are the 5 Colligative properties?
- Q. Why Colligative properties are called so?
- Q. What is Raoult’s Law used for?
- Q. What is a nonvolatile solute?
- Q. What are Colligative properties of water?
- Q. How do you calculate Molality?
- Q. Is color a Colligative property?
- Q. How do we use Colligative properties in everyday life?
- Q. How does osmosis affect mass?
- Q. How does osmosis affect weight?
- Q. How do you measure osmosis?
- Q. What is Plasmolysis and give an example?
- Q. What are the 3 types of phagocytes?
- Q. What are examples of phagocytes?
- Q. What are the 4 steps of phagocytosis?
- Q. What are the 7 steps of phagocytosis?
- Q. What is phagocytosis and its steps?
- Q. What occurs during phagocytosis?
- Q. How do phagocytes know what to eat?
- Q. How do phagocytes move?
- Q. Is phagocytosis good or bad?
- Q. Which blood cell is not phagocytic?
- Q. What happens during an inflammatory response?
- Q. Which cells do not perform phagocytosis?
- Q. How do phagocytes recognize foreign cells or bacteria?
Q. What is it called when a cell loses water and shrinks?
Plasmolysis is when plant cells lose water after being placed in a solution that has a higher concentration of solutes than the cell does. This is known as a hypertonic solution. This causes the protoplasm, all the material on the inside of the cell, to shrink away from the cell wall.
Q. What is hypotonic and hypertonic solution?
A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. If the solute concentration outside the cell is lower than inside the cell, and the solutes cannot cross the membrane, then that solution is hypotonic to the cell.
Q. How is isotonic defined?
1 : of, relating to, or being muscular contraction in the absence of significant resistance, with marked shortening of muscle fibers, and without great increase in muscle tone — compare isometric. 2 : isosmotic —used of solutions.
Q. What is isotonic to blood?
A 0.9% NaCl solution is said to be isotonic: when blood cells reside in such a medium, the intracellular and extracellular fluids are in osmotic equilibrium across the cell membrane, and there is no net influx or efflux of water.
Q. What is the isotonic value?
The term isotonic means equal tone, and is used interchangeably with isosmotic with reference to specific body fluids. For example, a 0.9% w/v solution of Nacl in water is considered to be isotonic in relation to RBC’s and their semi-permeable membranes.
Q. How is isotonic value calculated?
Calculations for preparation of isotonic solution: multiply the quantity of each drug in the prescription by it’s sodium chloride equivalent E , and subtract this value from the concentration of sodium chloride which is isotonic with body fluids (0.9 gm per 100 ml).
Q. What is the isotonic point of a potato?
– The isotonic point of the potato will be around 0.4 molecular concentration because potatoes are more moist than other vegetables. Assuming that 0.4 mc is the isotonic point, the concentration lower than 0.4 would be a hypotonic solution, making the weight of the potato increase.
Q. Which one is the same in isotonic solution?
Therefore, isotonic solutions are solutions having the same osmotic pressure. Hence, option C is the required answer.
Q. What are normal solutions?
It is similar to molarity but uses the gram-equivalent weight of a solute in its expression of solute amount in a liter (L) of solution, rather than the gram molecular weight (GMW) expressed in molarity. A 1N solution contains 1 gram-equivalent weight of solute per liter of solution.
Q. What is unit of molality?
The units of molality are m or mol/kg.
Q. Do isotonic solutions have same density?
Isotonic solutions must have the same………. (a) solute (b) density (c) elevation in boiling point (d) depression in freezing point. Answer: As the molar concentration is same for isotonic solutions, so elevation in boiling point and depression in freezing point of isotonic solutions must be same.
Q. What is molarity of pure water?
Now, we know that the volume of the solution is 1 L, the molarity of the solution is calculated by: Molarity=Moles of the soluteVolume of the solution=55.551=55.55 M. So the Molarity of pure water is 55.55 M.
Q. Is blood plasma isotonic?
The osmolarity of normal saline, 9 grams NaCl dissolved in water to a total volume of one liter, is a close approximation to the osmolarity of NaCl in blood (about 290 mOsm/L). Thus, normal saline is almost isotonic to blood plasma.
Q. Is isotonic solutions have same boiling point?
Isotonic solutions have same osmotic pressure and same concentration. Elevation in boiling point and d epression in freezing point are the colligative properties. As the molar concentration is same for isotonic solutions, so elevation in boilling point and depression in freezing point of istonic solutions must be same.
Q. Which is the highest boiling point?
Carbon has the highest melting point at 3823 K (3550 C) and Rhenium has the highest boiling point at 5870 K (5594 C).
Q. Which is not a Colligative property?
Depression in freezing point is a colligative property but freezing point is not a colligative property.
Q. What is meant by Colligative property?
Colligative properties of solutions are properties that depend upon the concentration of solute molecules or ions, but not upon the identity of the solute. Colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Q. What are the 4 Colligative properties?
There are four colligative properties: vapor pressure lowering, boiling point ele- vation, freezing point depression, and osmotic pressure. This means that a solution shows a decreased vapor pressure, an increased boiling point and a decreased freez- ing point in comparison to the pure solvent (water in our case).
Q. What are the 5 Colligative properties?
These colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Q. Why Colligative properties are called so?
These properties are called colligative properties; the word colligative comes from the Greek word meaning “related to the number,” implying that these properties are related to the number of solute particles, not their identities.
Q. What is Raoult’s Law used for?
Raoult’s law is a phenomenological law that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules: the conditions of an ideal solution.
Q. What is a nonvolatile solute?
A non-volatile substance refers to a substance that does not readily evaporate into a gas under existing conditions. Non-volatile substances exhibit a low vapor pressure and a high boiling point. Sugar and salt are examples of non-volatile solutes. Examples of volatile substances include alcohol, mercury, and gasoline.
Q. What are Colligative properties of water?
Overview of colligative properties The colligative properties of solutions consist of freezing point depression, boiling point elevation, vapor pressure lowering and osmotic pressure.
Q. How do you calculate Molality?
Molality Calculations
- The concentration of a solution can be given in moles of solute dissolved per kilogram of solvent.
- Molality is given the symbol m.
- molality = moles of solute ÷ mass of solvent in kilograms m = n(solute) ÷ mass(solvent in kg)
Q. Is color a Colligative property?
The four colligative properties commonly encountered are boiling point elevation, freezing point depression, lowering of vapour pressure, and osmotic pressure. All of these properties are important in physiological and natural systems. Another non-colligative property is the color of a solution.
Q. How do we use Colligative properties in everyday life?
For example, adding a pinch of salt to a cup of water makes the water freeze at a lower temperature than it normally would, boil at a higher temperature, have a lower vapor pressure, and changes its osmotic pressure.
Q. How does osmosis affect mass?
During osmosis substances move from an area of high pressure to an area of low pressure. Therefore, when a chip is placed in a concentrated sucrose solution, it will lose mass because the chip has a higher concentration of water than the sucrose solution. Water will move out from the chip and the cell becomes flaccid.
Q. How does osmosis affect weight?
It is possible to observe the result of osmosis. Bags that contain fluids hypertonic to the environment should gain water and weight. Bags that contain fluids hypotonic to the environment should lose water and weight.
Q. How do you measure osmosis?
Instead of measuring osmosis by its potential energy, which is virtually impossible to do, we quantify osmosis by determining the pressure that would be just enough to counteract the movement of water caused by diffusion.
Q. What is Plasmolysis and give an example?
When a living plant cell loses water through osmosis, there is shrinkage or contraction of the contents of cell away from the cell wall. This is known as plasmolysis. Example – Shrinkage of vegetables in hypertonic conditions.
Q. What are the 3 types of phagocytes?
The main types of phagocytes are monocytes, macrophages, neutrophils, tissue dendritic cells, and mast cells. Other cells, such as epithelial cells and fibroblasts, may also engage in phagocytosis, but lack receptors to detect opsonized pathogens and are not primarily immune system cells.
Q. What are examples of phagocytes?
Phagocytes include white blood cells of the immune system, such as monocytes, macrophages, neutrophils, and mast cells. Dendritic cells (i.e. antigen-presenting cells) are also capable of phagocytosis. In fact, they are called professional phagocytes because they are effective at it.
Q. What are the 4 steps of phagocytosis?
There are four essential steps in phagocytosis: (1) the plasma membrane entraps the food particle, (2) a vacuole forms within the cell to contain the food particle, (3) lysosomes fuse with the food vacuole, and (4) enzymes of the lysosomes digest the food particle.
Q. What are the 7 steps of phagocytosis?
- Step 1: Activation of Phagocytic cells and Chemotaxis.
- Step 2: Recognition of invading microbes.
- Step 3: Ingestion and formation of phagosomes.
- Step 4: Formation of phagolysome.
- Step 5: Microbial killing and formation of residual bodies.
- Step 6: Elimination or exocytosis.
Q. What is phagocytosis and its steps?
The Steps Involved in Phagocytosis. Step 1: Activation of the Phagocyte. Step 2: Chemotaxis of Phagocytes (for wandering macrophages, neutrophils, and eosinophils) Step 3: Attachment of the Phagocyte to the Microbe or Cell. Step 4: Ingestion of the Microbe or Cell by the Phagocyte.
Q. What occurs during phagocytosis?
Phagocytosis is a process wherein a cell binds to the item it wants to engulf on the cell surface and draws the item inward while engulfing around it. The process of phagocytosis often happens when the cell is trying to destroy something, like a virus or an infected cell, and is often used by immune system cells.
Q. How do phagocytes know what to eat?
Recognition of suitable objects by the plasma membrane of the phagocyte initiates phagocytosis. Knowledge of serum proteins that coat objects rendering them recognizable is considerable, but understanding of the chemical basis of recognition is meager. The signals activated by recognition are also not known.
Q. How do phagocytes move?
The phagocytes move by a method called chemotaxis. When phagocytes come into contact with bacteria, the receptors on the phagocyte’s surface will bind to them. Some phagocytes then travel to the body’s lymph nodes and display the material to white blood cells called lymphocytes.
Q. Is phagocytosis good or bad?
Surface phagocytosis may be an important pre-antibody defense mechanism which determines whether an infection will become a disease and how severe the disease will become.
Q. Which blood cell is not phagocytic?
So, the correct answer is ‘Lymphocytes’.
Q. What happens during an inflammatory response?
The inflammatory response (inflammation) occurs when tissues are injured by bacteria, trauma, toxins, heat, or any other cause. The damaged cells release chemicals including histamine, bradykinin, and prostaglandins. These chemicals cause blood vessels to leak fluid into the tissues, causing swelling.
Q. Which cells do not perform phagocytosis?
So, the correct answer is ‘Basophil’.
Q. How do phagocytes recognize foreign cells or bacteria?
How do phagocytes recognize foreign cells or bacteria? The phagocytes recognize molecules on pathogens not normally found on body cells. The foreign cells or bacteria secrete chemicals that the phagocytes recognize. The phagocytes recognize molecules on pathogens not normally found on body cells.