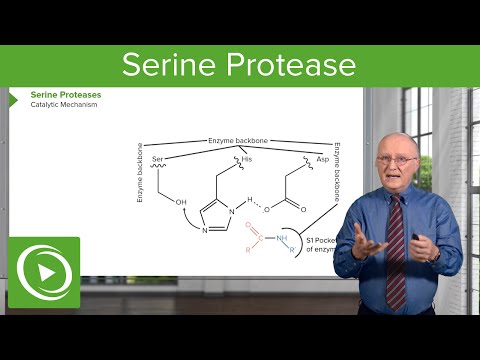
Q. What is the role of serine and histidine at the active site of serine proteases?
The serine has an -OH group that is able to act as a nucleophile, attacking the carbonyl carbon of the scissile peptide bond of the substrate. A pair of electrons on the histidine nitrogen has the ability to accept the hydrogen from the serine -OH group, thus coordinating the attack of the peptide bond.
Q. What are the functions of the three residues in the catalytic triad of serine proteases?
These three amino acids are often referred to as a catalytic triad. As the serine oxygen attacks the carbonyl carbon of a peptide bond, the hydrogen-bonded His functions as a general base to abstract the serine proton, and the negatively charged Asp stabilizes the positive charge that forms on the His residue.
Table of Contents
- Q. What is the role of serine and histidine at the active site of serine proteases?
- Q. What are the functions of the three residues in the catalytic triad of serine proteases?
- Q. Which residue in the catalytic triad is really responsible for the activity of serine proteases?
- Q. Why is the serine at the active site of chymotrypsin so reactive?
- Q. Which feature of chymotrypsin stabilizes the transition states?
- Q. Which phase of chymotrypsin mechanism is slower?
- Q. Where does chymotrypsin take action?
- Q. Why does chymotrypsin specificity pocket accommodate bulky aromatic side chains?
- Q. Where is chymotrypsin found in the body?
- Q. What is the significance of chymotrypsin?
- Q. What causes chymotrypsin?
- Q. What is the difference between trypsin and chymotrypsin?
- Q. What is the function of trypsin and chymotrypsin?
- Q. What is the function of trypsin?
- Q. Is trypsin a lock and key?
- Q. What amino acids do trypsin and chymotrypsin cut at?
- Q. Is trypsin a hydrolase?
- Q. Is trypsin a lyase?
- Q. Does trypsin activate Pepsinogen?
- Q. Why trypsin is released in inactive form?
- Q. Is trypsin a carboxypeptidase?
- Q. What is the difference between aminopeptidase and carboxypeptidase?
- Q. Does carboxypeptidase digest proteins?
- Q. What happens when zinc is removed from carboxypeptidase?
- Q. What is the function and releasing site of carboxypeptidase?
- Q. How is Zn bound in CPA?
- Q. Which one of the drug requires zinc in the active site of the enzyme?
Q. Which residue in the catalytic triad is really responsible for the activity of serine proteases?
9 Catalytic Triad. The serine esterases have a catalytic triad: serine, glutamic or aspartic acid, and histidine. These catalytic residues are responsible for the nucleophilic attack of the active site serine on the carbonyl carbon atom of the ester.
Q. Why is the serine at the active site of chymotrypsin so reactive?
The histidine was in position to act as a base, a proton acceptor, and remove the proton from the OH group of serine. With this change, the serine is much more reactive, and can easily form a new bond with the carbon atom in the peptide bond of the substrate.
Q. Which feature of chymotrypsin stabilizes the transition states?
The Oxyanion Hole. The structure stabilizes the tetrahedral intermediate of the chymotrypsin reaction. Hydrogen bonds (shown in green) link peptide NH groups and the negatively charged oxygen.
Q. Which phase of chymotrypsin mechanism is slower?
The second phase of the catalysis by chymotrypsin is slower. It requires that the covalent bond between phenylalanine and serine’s oxygen be broken so the peptide can be released and the enzyme can return to its original state. The process starts with entry of water into the active site.
Q. Where does chymotrypsin take action?
Chymotrypsin is a digestive enzyme synthesized in the pancreas that plays an essential role in proteolysis, or the breakdown of proteins and polypeptides. As a component in the pancreatic juice, chymotrypsin aids in the digestion of proteins in the duodenum by preferentially cleaving peptide amide bonds.
Q. Why does chymotrypsin specificity pocket accommodate bulky aromatic side chains?
(There will be eight steps.) : Chymotrypsin cleaves peptide bonds after bulky or aromatic side chains, such as those of the amino acids phenylalanine or tyrosine. The specificity pocket, or substrate-binding site, is deep and has hydrophobic side chains.
Q. Where is chymotrypsin found in the body?
pancreas
Q. What is the significance of chymotrypsin?
Trypsin:chymotrypsin is an oral proteolytic enzyme preparation which has been in clinical use since the 1960s. It provides better resolution of inflammatory symptoms and promotes speedier recovery of acute tissue injury than several of the other existing enzyme preparations.
Q. What causes chymotrypsin?
It typically occurs as a result of progressive pancreatic damage. A chymotrypsin test may be ordered when you have signs and symptoms of pancreatic insufficiency, such as: Persistent diarrhea. Abdominal cramps.
Q. What is the difference between trypsin and chymotrypsin?
Trypsin and chymotrypsin are two very similar digestive enzymes that hydrolyze proteins into amino acids. This is the main difference between these two enzymes. Activation: The inactive form of trypsin, trypsinogen, is activated by enterokinase, while chymotrypsinogen is activated by trypsin.
Q. What is the function of trypsin and chymotrypsin?
Trypsin and chymotrypsin are important digestive enzymes that are secreted by the pancreas as the inactive enzyme precursors trypsinogen and chymotrypsinogen. Trypsin activates itself via positive feedback and converts chymotrypsinogen and other inactive enzymes into their active forms.
Q. What is the function of trypsin?
Trypsin is an enzyme that helps us digest protein. In the small intestine, trypsin breaks down proteins, continuing the process of digestion that began in the stomach. It may also be referred to as a proteolytic enzyme, or proteinase. Trypsin is produced by the pancreas in an inactive form called trypsinogen.
Q. Is trypsin a lock and key?
The reaction that it catalyses is not necessarily specific to that particular peptide bond, but the trypsin selectively binds at that site. This role of specific binding in enzymes is often referred to as the “lock and key” mechanism.
Q. What amino acids do trypsin and chymotrypsin cut at?
Trypsin cuts at lysine and arginine, while chymotrypsin. cuts at tyrosine, phenylalanine, and tryptophan..
Q. Is trypsin a hydrolase?
Trypsin is a naturally occurring proteolytic enzyme found in the digestive tract of mammals. When converted from its zymogen trypsinogen, trypsin is available as an active peptide hydrolase (EC 3.4. 21.4) form to cleave peptide chains, mainly at the carboxyl side of the amino acids lysine or arginine.
Q. Is trypsin a lyase?
Trypsin (EC 3.4. 21.4) is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated.
Q. Does trypsin activate Pepsinogen?
Activation: The inactive form of pepsin, pepsinogen, is activated by HCl of the gastric juice, whilst the inactive form of trypsin, trypsinogen, is activated by an enzyme called enterokinase.
Q. Why trypsin is released in inactive form?
Trypsin is produced, stored and released as the inactive trypsinogen to ensure that the protein is only activated in the appropriate location. Premature trypsin activation can be destructive and may trigger a series of events that lead to pancreatic self-digestion.
Q. Is trypsin a carboxypeptidase?
The exocrine pancreas secretes three endopeptidases (trypsin, chymotrypsin, and elastase) and two exopeptidases (carboxypeptidase A and carboxypeptidase B) in inactive forms. Trypsin, for example, cleaves the peptide bonds in which basic amino acids (lysine and arginine) contribute the carboxyl group.
Q. What is the difference between aminopeptidase and carboxypeptidase?
is that carboxypeptidase is (enzyme) any enzyme that catalyzes the hydrolysis of the terminal amino acid of a polypeptide / protein from the end that contains a free carboxyl group while aminopeptidase is (enzyme) any of several enzymes that catalyze the hydrolysis of the peptide bond of the terminal amino acid at the …
Q. Does carboxypeptidase digest proteins?
To break down a protein into its constituent amino acids, the cell uses a hydrolysis reaction. The enzyme carboxypeptidase A is secreted by the pancreas and is used to speed up this hydrolysis reaction. As seen in Figure 2, this enzyme consists of a single chain of 307 amino acids.
Q. What happens when zinc is removed from carboxypeptidase?
CARBOXYPEPTIDASE is a metallo-enzyme which contains zinc. It has been reported by Coleman and Vallee1 that the enzyme can be de-activated by removal of zinc and that activity can be restored by the addition of zinc, cobaltous, ferrous, nickel and manganous ions but not by addition of cadmium, magnesium or calcium ions.
Q. What is the function and releasing site of carboxypeptidase?
A carboxypeptidase (EC number 3.4. 16 – 3.4. 18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins.
Q. How is Zn bound in CPA?
CPA-1 and CPA-2 (and, it is presumed, all other CPAs) employ a zinc ion within the protein for hydrolysis of the peptide bond at the C-terminal end of an amino acid residue. Loss of the zinc leads to loss of activity, which can be replaced easily by zinc, and also by some other divalent metals (cobalt, nickel).
Q. Which one of the drug requires zinc in the active site of the enzyme?
3.1 Physiological Functions of Zinc A classic example is alcohol dehydrogenase (ADH), an important enzyme in alcohol metabolism, which contains four zinc ions per molecule with two required for catalytic activity and the other two for protein confirmation.