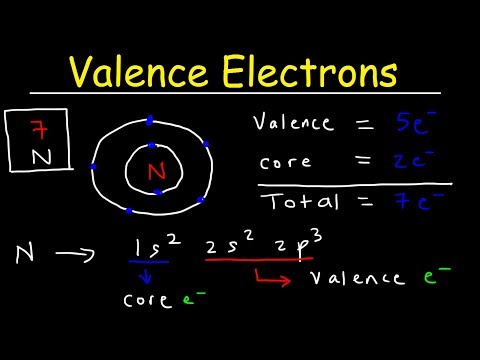
Q. What is the valence electrons of V?
The vanadium number of valence electrons is 5 and it belongs to the 5th group.
Q. What is the valence electron configuration of vanadium?
Ar 3d3 4s2
Table of Contents
- Q. What is the valence electrons of V?
- Q. What is the valence electron configuration of vanadium?
- Q. How do you find the valence of vanadium?
- Q. How do you calculate valence electrons?
- Q. How many valence electrons does group 13 have?
- Q. Which group on the periodic table is an atom with 5 valence electrons?
- Q. Which is the lightest element in the world?
- Q. Which element has a valency of 5?
- Q. What is the only metalloid with 7 valence electrons?
- Q. Which element has 4 energy levels and 7 valence electrons?
- Q. What elements has 4 energy levels?
- Q. Which elements has 2 valence electrons?
- Q. What Period 2 element has 4 valence electrons?
- Q. Does magnesium have 2 valence electrons?
- Q. What is the relationship between group number and valence electrons?
- Q. What does knowing the group number tell you about an element’s valence electrons?
- Q. What is the difference between Valency and group number?
- Q. What will an atom do if it has 1 to 3 valence electrons?
- Q. Do all transition metals have 2 valence electrons?
- Q. What is the best definition of valence electrons Quizizz?
- Q. When K+ and Cl meet they will?
- Q. What does KCl actually stand for?
- Q. What is the formula of K and Cl?
- Q. What is K formula?
- Q. How do you get KCl?
Q. How do you find the valence of vanadium?
Vanadium is a group 5 transition metal with d orbitals, but there’s a simple answer and a better answer. The simple answer is “+2, because it has the same +2 p orbital as the type 2 metals.” The better answer is “It depends, because d orbitals are strange. -1, +1, +2, +3, +4, and +5 are all possible oxidation states.”
Q. How do you calculate valence electrons?
For neutral atoms, the number of valence electrons is equal to the atom’s main group number. The main group number for an element can be found from its column on the periodic table. For example, carbon is in group 4 and has 4 valence electrons. Oxygen is in group 6 and has 6 valence electrons.
Q. How many valence electrons does group 13 have?
3
Q. Which group on the periodic table is an atom with 5 valence electrons?
group 15
Q. Which is the lightest element in the world?
Hydrogen
Q. Which element has a valency of 5?
Valency of First 30 Elements
| Element | Atomic Number | Valency |
|---|---|---|
| Valency of Helium | 2 | 0 |
| Valency of Lithium | 3 | 1 |
| Valency of Beryllium | 4 | 2 |
| Valency of Boron | 5 | 3 |
Q. What is the only metalloid with 7 valence electrons?
The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Halogens have 7 valence electrons, which explains why they are the most active non-metals.
Q. Which element has 4 energy levels and 7 valence electrons?
| Element | Element Number | Number of Electrons in each Level |
|---|---|---|
| Beryllium | 4 | 2 |
| Boron | 5 | 3 |
| Carbon | 6 | 4 |
| Nitrogen | 7 | 5 |
Q. What elements has 4 energy levels?
Since potassium, calcium, and bromine are in the 4th row or period, their outermost electrons are in the fourth energy level.
Q. Which elements has 2 valence electrons?
A: Calcium is a group 2 element with two valence electrons.
Q. What Period 2 element has 4 valence electrons?
Calcium
Q. Does magnesium have 2 valence electrons?
Valence electrons are the electrons in the outermost principal quantum level of an atom. Sometimes, the outermost energy level is called the valence shell. Therefore, magnesium has two valence electrons.
Q. What is the relationship between group number and valence electrons?
The number of valance electrons is equal to the group number of the representative elements. The relation between the group number and valance electrons is that “group number is equal to the number of valance electrons”.
Q. What does knowing the group number tell you about an element’s valence electrons?
The group number tells you the total number of electrons present in the outermost orbit of an atom. In other words, group number tells you the number of valence electrons of an atom. For example, Let us consider group 1 of the Periodic table.
Q. What is the difference between Valency and group number?
For groups 1-2 and 13-18, the valency is directly related to group number. So, elements in group one, the alkali metals, have one valence electron, those in group two have two valence, those in group thirteen have three electrons, and so on.
Q. What will an atom do if it has 1 to 3 valence electrons?
Answer and Explanation: If an atom needs 1 to 3 valence electrons, it will lose them to become a charged ion and form an ionic bond with another atom.
Q. Do all transition metals have 2 valence electrons?
2 Answers. Most transition metals have 2 valence electrons. Valence electrons are the sum total of all the electrons in the highest energy level (principal quantum number n).
Q. What is the best definition of valence electrons Quizizz?
Q. What are valence electrons? empty spaces in the outermost shell of an atom. electrons in the outermost shell available for bonding.
Q. When K+ and Cl meet they will?
Imagine what happens when a potassium (K) atom meets a chlorine (Cl) atom. The K atom will lose one electron and acquire a positive charge, so it will become a positive ion with a charge of +1, written as K+.
Q. What does KCl actually stand for?
potassium chloride
Q. What is the formula of K and Cl?
KCl
Q. What is K formula?
The Coulomb constant, the electric force constant, or the electrostatic constant (denoted ke, k or K) is a proportionality constant in electrostatics equations. In SI units it is equal to 8.9875517923(14)×109 kg⋅m3⋅s−2⋅C−2.
Q. How do you get KCl?
Preparation: KCl is commonly obtained by mining its minerals, followed by extraction. It is also extracted from brine (salt water). It can also be prepared in the laboratory in small scales by reacting potassium hydroxide (KOH) with hydrochloric acid (HCl).